By Quratulain Khan1, Bushra Wasim1, Kanwal Haneef2, Shumaila Usman3
AFFLIATIONS:
- Department of Anatomy, Ziauddin University, Karachi, Pakistan.
- Zafar H. Zaidi Centre for Proteomics, Karachi University, Karachi, Pakistan.
- Department of Research, Ziauddin University, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-3/009
ORCID iD: 0000-0002-9560-8099
How to cite: Khan Q, Wasim B, Haneef K, Usman S. Isolation of Homogenous Population of Human Amniotic Epithelial Stems Cells. Pak J Med Dent. 2022;11(3): 51-58. doi: 10.36283/PJMD11-3/009
Background: Regenerative medicine is a collaborative field that applies combined concepts of tissue engineering and cell biology to promote regeneration. Tissue regeneration initiate the emergence of remedies that can replace and revive tissues and loss of organs through illness and injury and reduce dependency on organ transplantation. The objective was to isolate a homogenous population of human amniotic epithelial stem cells (hAECs) and to determine their phenotype under a live-cell imaging microscope at 1st, 3rd and 8th-day intervals.
Methods: This was an experimental in-vitro study conducted on human placenta (amniotic epithelial stem cells) at Ziauddin Hospital and University. The 22 human placenta were collected from patients with age 20-30 years having healthy full-term pregnancies, with a preference for elective cesarean sections. In vitro isolation of human amniotic epithelial stem cells was used after manual removal of the amniotic membrane from the chorionic membrane of human term placenta via single-step enzymatic digestion. The morphology of the attached cells was analyzed at different magnifications i.e., 10X, 20X and 40X.
Results: The isolated human amniotic epithelial stem cells on day 3 started forming colonies and attained a characteristic cuboid epithelial shape and exhibited a confluent single layer on day 8. Morphologically resembled cobblestones with a characteristic of fully vacuolated cytoplasm were found uncontaminated with spindle-shaped human amniotic mesenchymal stem cells (MSCs).
Conclusion: The isolated population of hAESCs were highly proliferative and not contaminated with MSCs. Therefore, this optimized protocol may be used for the isolation and the biobanking of hAESCs for future cellular transplantation.
Keywords: Human Amniotic Epithelial Stem Cells (hAESCs); Regenerative Medicine; Perinatal Stem Cells; Placental Membrane; Amniotic Membrane.
The understanding of regenerative medicine is based on the capability to regenerate and substitute impaired tissues and organs. Regenerative medicine has revealed encouraging outcomes for the renewal and substitution of multiple tissues and organs such as skin, heart, kidney, and liver and the ability to even correct some inborn defects. The treatment of patients, as well as scientific research, is delayed due to the scarcity of tissues and organs. Regenerative medicine may assist in solving this tasks1.
Based on sources of stem cells, they can be classified into four categories: embryonic, induced pluripotent, perinatal, and adult stem cells2. Adult stem cells are in the organs or tissues at maturity. They can differentiate into mature cell types and play their part in tissue regeneration. Embryonic stem cells (ESCs), derived from the embryo blast (inner cell mass) of the blastocyst, are pluripotent, being able to differentiate into the structures of three germ layers and being capable of self-renewal3,4. Whereas induced pluripotent stem cells which were firstly introduced by Dr. Yamanaka in 2006, can be used, as an alternative to ESCs, with a demerit to develop teratoma5. Nowadays, researchers and clinicians are exploring a novel cell type with less tumorigenicity, increased differential potential and no ethical concern6. Perinatal sources such as the placental membranes, umbilical cord and amniotic fluid have gained attention as a potential source of stem cells for regenerative medicine7.
The human placenta is a biological connection between the fetus and the mother and is meant to perform important functions like nutrition, respiration and excretion8. Fetal membranes surrounding the fetus comprise an amniotic and chorionic membrane developed from the inner cell mass of the blastocyst. Amnion which is an avascular layer, in connection with amniotic fluid, obtains its nutrients through diffusion. Microscopically, the amnion constitutes a layer of epithelial stem cells and a thick basement membrane9. A large scale of research concentrated on cell-derived from the amniotic membrane as it is cost-effective has minimal ethical issues and could be retrieved non-invasively. Human amniotic epithelial stem cells (hAESCs) express markers of pluripotency such as SSEA-4, OCT-4, and NANOG. hAESCs have high differentiation potential into all the three germ layers’ derivatives including hepatocytes, pancreatic beta cells, neurons, cardiomyocytes, chondrocytes and osteocytes10,11. Besides pluripotency, hAESCs are characterized as an outstanding applicant for allogeneic cell transplantation as hAESCs release a wide range of anti-inflammatory cytokines, reduce fibrosis express HLA G and low levels of telomerase12-14. Therefore, researchers apply hAESCs in pre-clinical trials for the management of a wide variety of diseases including stroke, brain injury, Parkinson’s disease, atherosclerosis, myocardial infarction, dentistry and ophthalmology14-16.
By investigating hAESCs behavior in proliferation and survival, clinicians would be able to assess their reliable healing potential in different degenerative disorders. Biobanking of hAESCs could also be done for a future cellular transplantation approach which brings its application closer to reality. It will move research from the bench to the bedside and will also contribute valuable information for clinicians and future investigators. Therefore, this study aimed to isolate a homogenous population of hAESCs that has anti-inflammatory and immunomodulatory properties as well as non-tumorigenic potential and to determine their phenotype under live-cell imaging microscope at 10X, 20X and 40X magnifications on 1st, 3rd and 8th day intervals
In this study, the 22 human placenta was collected from patients with age 20-30 years having healthy full-term pregnancies, with a preference for elective cesarean sections. Placenta was collected after taking written and informed consent from the patients admitted in the Kemari, Clifton, and North Nazimabad branches of Ziauddin hospital. Isolation was done at the University of Karachi. Patients with maternal diabetes, hypertension, thyroid abnormalities, placenta previa, placenta abruption, and abnormality in the fetus detected on scanning during the antenatal period were not included in the study.
After approval from the institutional ethics committee, this experimental in-vitro study was conducted on the human placenta (amniotic epithelial stem cells to retrieve amniotic epithelial stem cells. Every experiment was conducted in triplicate. The full-term placenta was received after an elective cesarean section. The amniotic membrane was manually separated from the chorionic layer, starting from the reflected edge of the fetal membranes towards the umbilical cord. A 7cm long piece of amnion was cut and placed in a 50 ml of falcon tube which has 20 ml of PBS to remove large blood clots. The amnion was transferred into another 50 ml falcon containing 20 ml PBS, to wash off the remaining blood and debris. The process was repeated thrice and then the sample was shifted to the stem cell culture lab (Figure 1).
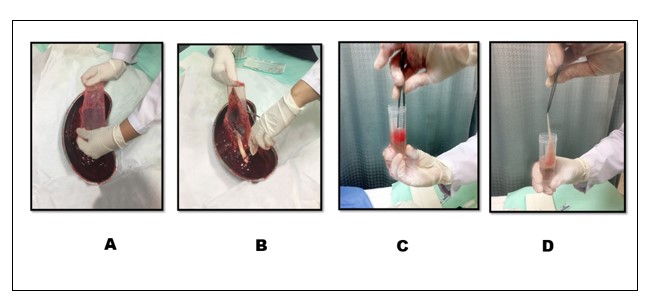
Figure 1: Full term placenta was received in kidney tray after elective cesarean sections. A. Starting from the reflected edge of the placenta avascular amniotic membrane separated from the chorion toward the umbilical cord. B. Cutting of amniotic membrane C. Amnion was placed in 50 ml of falcon tube containing 20 ml of PBS with the help of sterile forceps to wash the amnion and remove the large blood clots. D. The cleared amniotic membrane sample was shifted to the stem cell culture lab.
The human amniotic epithelial cells were isolated in class II biosafety cabinets according to the following optimized protocol (Figure 2). The 7cm long piece of amnion was washed thrice with 10ml PBS in 100 mm Petri plates. After washing, amnion was cut into eight pieces and transferred into 3 ml of 10X trypsin for enzymatic digestion. Four pieces of amnion were transferred to each Petri plate. The amnion-containing plates were manually shaken for five minutes and then, the plates were incubated at 37oC with 5% CO2 for 30 minutes. After incubation, the gel was removed from each piece of amnion with the help of two sterile forceps and 4 ml of complete DMEM medium was added to each plate. The amnion gel was homogenized using pipetting and then, cells were observed under a live cell imaging microscope. The medium containing hAESCs was transferred into three 25cm2cell culture flasks. The isolated hAESCs were cultured in DMEM (Gibco, Life Technologies) containing 10% fetal bovine serum (FBS) (MP Biomedical Inc), penicillin/streptomycin, sodium pyruvate and L-glutamine, at 37°C in 5% CO2 incubator. The medium was changed after every three days till cells become 70% confluent.
The isolated cells were observed daily under a live cell imaging microscope at different time intervals to observe the cell attachment and colony formation of the cells. The morphology of the attached cells was analyzed at different magnifications i.e., 10X, 20X and 40X.
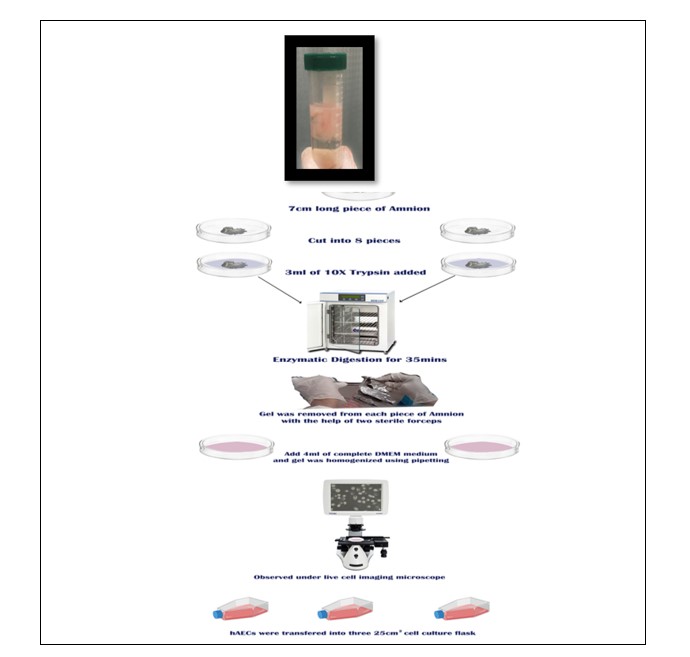
Figure 2: Diagrammatic representation of isolation of human amniotic epithelial cells (hAECs).
Human amniotic epithelial stem cells (hAESCs) were visualized under a live-cell imaging microscope at different time intervals and different magnifications. On day 1, amniotic epithelial stem cells were trapped in the gel and some were free-floating, on day 3hAESCs were attached to the flask and started forming colonies whereas, 70% confluency was attained on the 8th day of isolation (Figure 3).
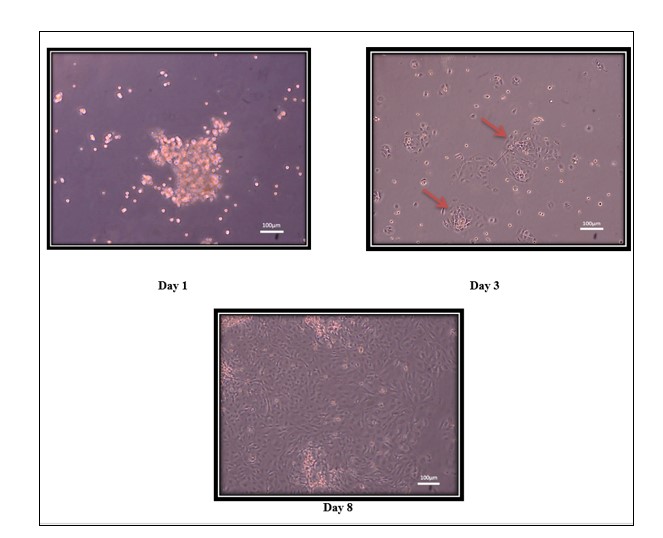
Figure 3: Homogenous Population Of hAECs Human amniotic epithelial cells was visualized under a live-cell imaging microscope at 10X magnification. (A) Day 1 with amniotic epithelial cells trapped in the gel. (B) Amniotic epithelial cells on day 3 attached with flask and started forming colonies (marked by red arrows). (C) Human amniotic epithelial cells attain 70% confluence on day 8.
hAESCs cells displayed more rounded homogenous populations of human amniotic epithelial stem cells at 10X, Cobblestone morphology typical of hAESCs at 20X and hAESCs fully vacuolated cytoplasm at 40X magnification (Figure 4).
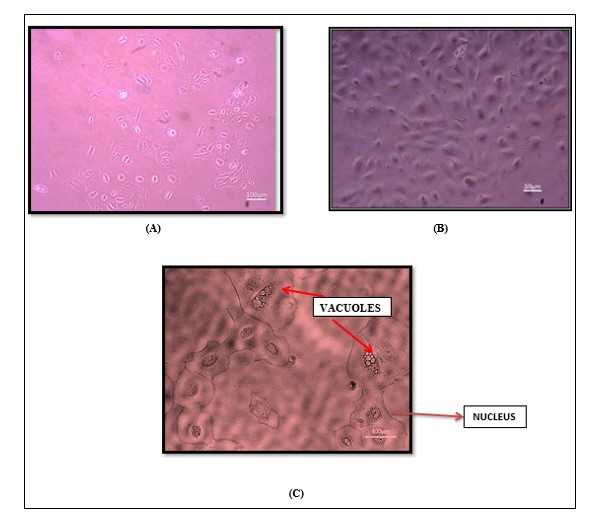
Figure 4: Morphological Examination of hAECs. hAECs were observed under a live-cell imaging microscope at different magnifications. (A) Homogenous populations of rounded human amniotic epithelial cells at 10X. (B) Cobblestone-like morphology was typical of hAECs at 20X. (C) Human amniotic epithelial cells fully vacuolated (red arrow) cytoplasm at 40X magnification.
This study optimized the isolation protocol and obtain rounded cobblestone-like pure human amniotic epithelial stem cells not contaminated with spindle-shaped MSCs. Perinatal stem cells are easily procured in an economical and non-invasive manner from tissues that are discarded at birth. Among them amniotic epithelial stem cells gain enhanced attention, are less characterized and are a beneficial source of stem cells that are utilized clinically to treat chronic diseases14,17,18.
However, there was great variation in cell yield in different isolation protocols, which was generally attributed to the quality of tissue (amniotic membrane) received, pre isolation time (tissue processing time), type and concentration of enzyme, steps and time for tissue digestion19-21. These are the vital parameters that had an important impact on the successful isolation of cells. The quality of the amniotic membrane is determined by proper washing, tissue processing time and the mode of delivery. Since amnion obtained from elective cesarean section (ELSC) has fewer chances of contamination than normal vaginal delivery22. Therefore, the amniotic membrane of elective cesarean sections was collected. Proper washing of the tissue is mandatory for tissue digestion. Since the RBCs, as well as serum, may inhibit the trypsin efficiency19,23. Therefore, sterile PBS with antibiotics was used for proper washing of the amniotic membrane. The washing steps were repeated until the tissue appeared transparent. Another main determinant affecting the quality of tissue is the time between collection of amniotic membrane and initiation of the isolation procedure (tissue processing time)20. Earlier studies reported that the amnion collected from the hospital or center should be transferred to the culture lab as soon as possible to minimize the pre isolation time or tissue processing time because prolonged storage or increased pre isolation time may decline the cell viability19,24.
The epithelial monolayer, a thick underlying membrane, and an avascular stroma make up the structure of the amniotic membrane. A single layer of epithelial stem cells was organized uniformly on the basement membrane, composed of a compact layer of cuboidal and columnar epithelial stem cells. Both human amniotic epithelial stem cells and human amniotic mesenchymal stem cells were successfully isolated from amnion, exhibiting cobblestone-shape and fibroblast-like morphology respectively15.
Motedayyen et al. reported high yield and viable hAESCs following the amniotic membrane digestion thrice with 1X trypsin. However, the isolated cells were contaminated with MSCs20. Another study reported a heterogeneous population of hAESCs when the cells recovered by second digestion because cells were trapped in the gel25. Murphy et al. stated that by decreasing tissue digestion time contamination with MSCs can be reduced. However, this may also decrease the total yield of cells19. The current study optimized the protocol for a homogenous population of hAESCs, without any contamination of MSCs and successfully obtained a higher yield of hAESCs. The isolation protocol after several modifications was designed. The protocol established for the isolation of pure hAESCs is based on two important factors i) shorter incubation time, and ii) one-step digestion. The isolation was started with previously reported protocols25,26. The isolation was started with two-step digestion and each digestion was for 30 minutes. However, sufficient hAESCs were not obtained. The next optimization step involved the digestion of the sample for 45 minutes. Still, the study failed to get enough cell population. Finally, the tissue was digested in 10X trypsin for 30 minutes. It was found that by shortened incubation time cellular yield was increased without any contamination. According to previous studies, shorter digestion of tissue increases the hAESCs yield26.
It was reported that 1X Trypsin resulted in reduced cell yield25. However, in the current study, the efficacy of 10X versus 1X concentrations in hAESCs isolation was assessed and evaluated with four separate amniotic membrane samples. The results showed that digestion of tissue in 1X Trypsin with two-step incubation failed in cell recovery. Regarding the truth that the efficiency of 10X trypsin in contrast to 1X Trypsin in hAESCs isolation is variable, therefore this protocol was optimized by digesting the membrane with 10X trypsin to obtain the maximum number of a viable and homogenous population of human amniotic epithelial stem cells27. The optimization of hAESCs isolation protocol within single digestion using 10X trypsin for 30 minutes was done.
It was found that hAESCs started forming colonies on day 3 which is a critical factor for the long-term survival of cells. Furthermore, cells continue to proliferate i.e., attain viability (cell attachment) at about 80% and confluency of culture flask at about 70% on the eighth day of isolation.
In other studies, they found specific enzymatic digestion of amniotic membrane in which human amniotic epithelial stem cells (hAESCs) and amniotic mesenchymal stem cells (hAMSCs) were digested by protease enzymes trypsin and collagenases respectively15,19,23,28. Human amniotic epithelial stem cells are in a single layer bound with weak cellular interaction so trypsin dissociates them easily20,27. The collagenase-based digestion solution was identified to cause stromal cell breakdown and that results in stromal cell release15,25.
The isolated population of human amniotic epithelial stem cells (hAESCs) in the study was highly proliferative and not contaminated with mesenchymal stem cells (MSCs). Therefore, the optimized protocol may be used for the isolation and biobanking of hAESCs, for a future cellular transplantation approach that will bring its application closer to reality.
This research was conducted at Ziauddin Medical University in collaboration with the University of Karachi (Proteomics Department).
There is no conflict of interest among the authors regarding the publication of this article.
The study was approved by the ethics review committee of the Ziauddin University, Karachi with the reference code: 1050419QKANA.
Informed consent was taken from patients. Patient identity was not disclosed at any point during the research.
This is a High Education Commission (HEC), Pakistan, funded the research project.
QK did the conception, and literature search and wrote the first draft of the manuscript. SU did the work design, analysis and interpretation of data and revised the draft critically. KH accountable for all aspects of the work ensuring that questions related to the accuracy of the work were investigated and resolved. BW did the proofreading and further finalized the article.
- Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, et al. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cell Int. 2018;2018:1-24. doi: 10.1155/2018/2495848
- Gaggi G, Izzicupo P, Di Credico A, Sancilio S, Di Baldassarre A, Ghinassi B. Spare parts from discarded materials: Fetal annexes in regenerative medicine. Int J Mol Sci. 2019;20(7):1-18. doi: 10.3390/ijms20071573
- Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, et al. Strategies to optimize adult stem cell therapy for tissue regeneration. Int J Mol Sci. 2016;17(6):1-16. doi:10.3390/ijms17060982
- Do EK, Moon HJ, Kang KT, Yoon JW, Kim YS, Seo JK, et al. Kap1 regulates the self-renewal of embryonic stem cells and cellular reprogramming by modulating Oct4 protein stability. Cell Death Differ. 2021;28(2):685-699. 10.1038/s41418-020-00613-x
- Zhou XB, Li H, Li F, Song XK, Liu T, Ma T, et al. Generation and characterization of two iPSC lines from human adipose tissue-derived stem cells of healthy donors. Stem Cell Res. 2020;48:1-4. doi: 10.1016/j.scr.2020.101973
- Abbaspanah B, Momeni M, Ebrahimi M, Mousavi SH. Advances in perinatal stem cells research: a precious cell source for clinical applications. Regene Med. 2018;13(05):595-610. doi: 10.2217/rme-2018-0019
- Li Z, Han ZC. Introduction of perinatal tissue-derived stem cells. InPerinatal Stem Cells 2019 (pp. 1-7). Springer, Singapore. doi: 10.1007/978-981-13-2703-2
- Knöfler M, Haider S, Saleh L, Pollheimer J, Gamage TK, James J. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell Mol Life Sci. 2019;76(18):3479-3496. doi: 10.1007/s00018-019-03104-6
- Huddleston HP, Cohn MR, Haunschild ED, Wong SE, Farr J, Yanke AB. Amniotic product treatments: clinical and basic science evidence. Curr Rev Musculoskelet Med. 2020;13(2):148-154. doi: 10.1007/s12178-020-09614-2
- Bollini S, Silini AR, Banerjee A, Wolbank S, Balbi C, Parolini O. Cardiac restoration stemming from the placenta tree: insights from fetal and perinatal cell biology. Front Physiol. 2018;9:385. doi: 10.3389/fphys.2018.00385
- Muttini A, Barboni B, Valbonetti L, Russo V, Maffulli N. Amniotic epithelial stem cells: Salient features and possible therapeutic role. Sports Med Arthrosc Rev. 2018;26(2):70-74. doi: 10.1097/JSA.0000000000000189
- Maymó JL, Riedel R, Pérez-Pérez A, Magatti M, Maskin B, Dueñas JL, et al. Proliferation and survival of human amniotic epithelial cells during their hepatic differentiation. PLoS One. 2018;13(1):1-28. doi: 10.1371/journal.pone.0191489
- Strom SC, Gramignoli R. Human amnion epithelial cells expressing HLA-G as novel cell-based treatment for liver disease. Hum Immunol. 2016;77(9):734-739. doi: 10.1016/j.humimm.2016.07.002
- Di Germanio C, Bernier M, De Cabo R, Barboni B. Amniotic epithelial cells: a new tool to combat aging and age-related diseases? Front Cell Dev Biol. 2016;4:1-8. doi: 10.3389/fcell.2016.00135
- Liu QW, Huang QM, Wu HY, Zuo GS, Gu HC, Deng KY, et al. Characteristics and therapeutic potential of human amnion-derived stem cells. Int J Mol Sci. 2021;22(2):1-31. doi: 10.3390/ijms22020970
- Nejad AR, Hamidieh AA, Amirkhani MA, Sisakht MM. Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta. 2021;103:104-119. doi: 10.1016/j.placenta.2020.10.026
- Ghamari SH, Abbasi-Kangevari M, Tayebi T, Bahrami S, Niknejad H. The bottlenecks in translating placenta-derived amniotic epithelial and mesenchymal stromal cells into the clinic: current discrepancies in marker reports. Front Bioeng Biotechnol. 2020;8:1-13. doi: 10.3389/fbioe.2020.00180
- Bejaoui M, Ferdousi F, Zheng YW, Oda T, Isoda H. Regulating cell fate of human amnion epithelial cells using natural compounds: An example of enhanced neural and pigment differentiation by 3, 4, 5-tri-O-caffeoylquinic acid. Cell Commun Signal. 2021;19(1):1-12. doi: 10.1186/s12964-020-00697-5
- Murphy SV, Kidyoor A, Reid T, Atala A, Wallace EM, Lim R. Isolation, cryopreservation and culture of human amnion epithelial cells for clinical applications. J Vis Exp. 2014(94):1-8. doi: 10.3791/52085
- Motedayyen H, Esmaeil N, Tajik N, Khadem F, Ghotloo S, Khani B, et al. Method and key points for isolation of human amniotic epithelial cells with high yield, viability and purity. BMC Res Notes. 2017;10(1):1-8. 10.1186/s13104-017-2880-6
- Dominguez RG. Human Amniotic Epithelial Cells: Isolation and Characterisation. VVB Laufersweiler; 2008. Available from: http://geb.uni-giessen.de/geb/volltexte/2015/11512/pdf/GomezDominguezRuth_2008_12_16.pdf
- Diema Konlan K, Baku EK, Japiong M, Dodam Konlan K, Amoah RM. Reasons for women’s choice of elective caesarian section in duayaw nkwanta hospital. J Pregnancy. 2019;2019:1-8. doi: 10.1155/2019/2320743
- Miki T, Marongiu F, Dorko K, Ellis EC, Strom SC. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2010;12(1):1-3. doi: 10.1002/9780470151808.sc01e03s12
- Gottipamula S, Sridhar KN. Large-scale isolation, expansion and characterization of human amniotic epithelial cells. Int J Stem Cells. 2018; 11(1): 87-95. doi: 10.15283/ijsc18001
- Gramignoli R, Srinivasan RC, Kannisto K, Strom SC. Isolation of human amnion epithelial cells according to current good manufacturing procedures. Curr Protoc Stem Cell Biol. 2016;37(1):1-13. doi: 10.1002/cpsc.2
- Naeem A, Gupta N, Arzoo N, Naeem U, Khan MJ, Choudhry MU, et al. A survey and critical evaluation of isolation, culture, and cryopreservation methods of human amniotic epithelial cells. Cell Cycle. 2022;21(7):655-673. doi: 10.1080/15384101.2021.2020015
- Schwartz RE, Trehan K, Andrus L, Sheahan TP, Ploss A, Duncan SA, et al. Modeling hepatitis C virus infection using human induced pluripotent stem cells. Proc Nat Acad Sci. 2012;109(7):2544-2558. doi: 10.1073/pnas.1121400109
- Deng J, Luo K, Xu P, Jiang Q, Wang Y, Yao Y, et al. High-efficiency c-Myc-mediated induction of functional hepatoblasts from the human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2021;12(1):1-6. doi: 10.1186/s13287-021-02419-1
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
