By Rozina Khatoon1, Habiba Sharaf Ali1, Ome Kulsoom1, Mehreen Yousaf Rana1, Monika Maheshwari2
AFFLIATIONS:
- Gynecologist, Karachi, Pakistan.
- Department of Health, Government of Sindh, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-3/010
ORCID iD: 0000-0001-7110-5414
How to cite: Khatoon R, Ali HS, Kulsoom O, Rana MY, Maheshwari M. Impact of Myo-Inositol on Ovary and Menstrual Cycle in Polycystic Ovarian Syndrome (PCOS) – A Therapeutic Approach. Pak J Med Dent. 2022;11(3): 59-65. doi: 10.36283/PJMD11-3/010
Background: Women of reproductive age develop menstrual irregularities along with infertility because of polycystic ovary syndrome (PCOS); the most common endocrine disorder. Myo-inositol (MI) is found to have a proven role in the treatment of this disorder. The objective of this study was to determine the efficacy of Myo-inositol in regulating the menstrual cycle in women with PCOS.
Methods: The study was conducted in 2019 at the Obstetrics and Gynecology Department of a tertiary care hospital with a sample of 50 women aged 18-45 years, having PCOS diagnosed with complaints of irregular menstrual cycles. Women with ovulatory dysfunction were excluded. Myo-inositol was given as 2 gm/day for 3 months. Pre- and post-trial data were collected, compared and analyzed through SPSS version 23 and a p-value <0.05 was considered statistically significant.
Results: Mean±SD age of participant women was 27.68 ± 4.787 years. The menstrual cycle duration increased from Mean±SD 4.36 ± 2.22 to 4.70 ± 1.51 days after treatment with MI. The cycle flow increased from 19.10 ± 37.92 to 14.12 ± 13.34 ml (p-value < 0.001 each). Right and left ovary volume also decreased (p-value < 0.001 each) post-treatment. There were significant improvements in cycle flow severity towards normal pattern (from 14% to 80%; p-value < 0.001). The efficacy of Myo-inositol in regulating menstrual flow severity increased with decreasing age (p-value = <0.0001).
Conclusion: Myo-Inositol was found efficacious in the treatment of PCOS and corrects menstrual irregularities, and menstrual flow, and normalizes ovarian volumes.
Keywords: Inositol; Polycystic Ovary Syndrome (PCOS); Menstrual Irregularities; Myo-inositol.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age. The hormonal and metabolic impairments lead to ovarian dysfunction and menstrual irregularities which are found in >50% of affected women1,2. The diagnosis requires the presence of features like; hyperandrogenemia, oligo/amenorrhea, menstrual irregularity, presence of 2-9 mm ovarian microcysts on ultrasound, >10 cm3 volume of ovaries and/ or insulin resistance (IR)3.
Over decades, PCOS has been treated through a variety of non-pharmacologic and pharmacological therapies however; the two stereoisomers of the Inositol family ~ Myo-inositol (MI) and D-chiro-Inositol (D-chiro-Ins), provided positive results4,5. These insulin sensitizer Inositols take part in process of insulin-mediated ovarian androgen synthesis (D-chiro-Ins) as well as facilitate the uptake of glucose and follicle-stimulating hormone signaling (Myo-Ins)6. Normally the ovary cells have an abundance of MI (~99%) and only traces of D-chiro-Ins7. It has been found that imbalance between the two in the ovary cells, leads to disruption of Follicle-stimulating hormone (FSH) signaling which is the hallmark of PCOS8.
The MI regulates the menstrual cycle within three months of therapy, is economically affordable, easy to administer, shorter duration of therapy and improves endocrinological problems. Like other treatment options for PCOS, it does not have any major side effects or interference with fertility9,10. Myo-inositol (MI) and D-chiro-Inositol (D-chiro-Ins)- are found effective in treating dysmenorrhea and other features of PCOS like correction of sizes of ovaries6. The three months of therapy with MI regulate ovarian volumes.
These Inositols isomers particularly MI is useful in PCOS treatment yet; there is very scant research conducted on the usefulness of the Myo-inositol in Pakistan. Therefore; the current study was commenced to determine the efficacy of MI in regulating the menstrual cycle and ovarian volume/size in PCOS and based on the results suggest use to help improve the menstrual irregularities in such women in the future.
A sample of 50 women who were diagnosed case of a polycystic ovarian syndrome, aged 18-45 years presenting with OPD with complaints of irregular menstrual cycle were included in the study consecutively. This experimental study with a duration of one year (01-01-2019 to 31-12-2019) was conducted at the Obstetrics and Gynecology Department of tertiary care hospital Karachi in 2019.
Polycystic ovary syndrome is defined as a medical disease of a women’s endocrine system manifesting as irregular menstrual cycles, chronic anovulation/ oligo or amenorrhea, androgen excess, and ultrasound features. Normal menstrual cycle duration was defined as 28 days with a range of 24-32 days. Menstrual blood volume was measured by asking women about the pads soaked during the menstrual cycle (Each fully soaked pad = 05ml). Accordingly, <20 ml was labeled as low, 21-50 ml as Normal while > 50 ml was labeled as heavy menstrual flow. Women having a history of ovulatory dysfunction, such as hyperprolactinemia or hypothyroidism, androgen excess, such as adrenal hyperplasia or Cushing’s syndrome were excluded. The study was performed with the permission of the Research Evaluation Committee of University. Written informed consent was obtained from the women. Detailed history regarding age, age of menarche, and menstrual history were taken. Ultrasound was performed to assess the size of the ovaries.
Blood investigations were done to obtain baseline information. Other specific investigation as per requirement were assessed on menstrual cycle while women hormonal profile was evaluated on any random day. Myo-Inositol (MI) therapy is used with a daily dose of 2000 mg of Myo-inositol for 3 months. Follow-up visit called at 3 months for clinical assessment of menstrual regularity and the size of ovaries. Both pre- and post-therapy data were compared to produce the results.
Data analysis was conducted through SPSS software, version 23. Mean ± SD was expressed for quantitative variables like age, BMI, marital status and qualitative variables like menstrual history, frequency and percentages were calculated. Categorical analysis was performed following the stratification of variables. A p-value <0.05 was deemed as significant. While for the continuous data the paired t-test <0.05 was applied to determine the efficacy of MI therapy in terms of regulation of menstrual cycle and flow.
The mean ± SD age of these women was 27.68 ± 4.787 years with a range from 19 to 38 years. It was noted that most patients were between the age of 21-30 years. Seventy percent (n = 35) of women were of the group aged 21 to 30 years (Figure 1). About three-fourths of all women (n = 38) were married.
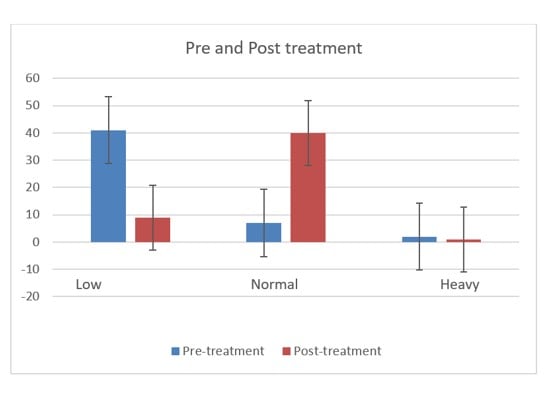
Figure 1: Comparison of pre- with post-Myo-inositol treatment flow severity.
The chi-square statistic is 43.9835. The p-value is < 0.00001.
Table 1 compared the characteristics of menstrual regularity (Cycle Duration, Cycle Flow) and ovarian volume before and after treatment with Myo-Inositol and shows a statistically significant better picture and regulation of normality after treatment. Post-treatment with the MI, there was a significant regulation of menstrual cycle duration, cycle flow as well as the normalization of ovarian volume was found.
Table1: Menstrual regularity characteristics described before and after Myo-inositol treatment.
| Characteristics | Minimum | Maximum | p-Value | ||
| Cycle Duration (days)
Mean ± S D |
Pre | Post | Pre | Post | <0.001 |
| 2 | 3 | 10 | 8 | ||
| 4.36 ± 1.22 | 4.70 ± 1.51 | ||||
| Cycle Flow (ml)
Mean ± S. D |
0 | 05 | 75 | 65 | <0.001 |
| 1.10 ±1.4 | 37.92 ± 13.34 | ||||
| Right Ovary volume: (cm) on U/S
Mean ± S. D |
3.9 | 10.9 | 5.8 | 15.5 | <0.001 |
| 7.89 ± 1.31 | 9.39 ± 3.40 | ||||
| Left Ovary volume: (cm3) on U/S
Mean ± S. D |
3.8 | 10.3 | 5.3 | 14.0 | <0.001 |
| 7.91 ±1.48 | 8.88 ±2.85 | ||||
* (p<0.001) highly significant as the cycle regulates towards the normal duration increase in lowest range while a decrease in the highest range of cycle duration while overall mean duration also increased.
* (p<0.001) highly significant as the cycle flow regulates towards the normal volume from higher as well as a lower range while overall mean flow also increased.
* (p<0.001) highly significant as the volume of both ovaries regulates towards normal.
Graphical presentation of categorical difference of MI treatment on the menstrual flow severity among treated women. There was an increase in the proportion of cases with a normal level of menstrual flow and a decrease in low as well as very high severity of menstrual flow cases. This finding was statistically very significant (p-value < 0.001). The student’s t-test revealed significant differences in the regulation of the menstrual cycle (duration, flow, ovarian volume and size) of participating women from pre-treatment to post-treatment (p <0.0001) given in Table 1.
The categorical analysis given in Table 2 showed a comparison of age, menstrual flow, BMI and volume of ovaries as effect modifiers. The relationship of difference between these variables with the pre- and post-Myo-inositol treatment is significant (p-value < 0.0001).
Table 2: Comparison of the effect of age of women on menstrual flow severity with pre-and post- Myo-inositol treatment.
| Parameters | Pre-treatment | Post-treatment | p-Value | |
| Age in Years
n (%) |
18-20 | 2 (4%) | 2 (4%) | NA |
| 21-30 | 35 (70%) | 35 (70%) | ||
| 31-40 | 13 (26%) | 13 (26%) | ||
| Normal | 7 (14%) | 40 (80%) | ||
| Heavy | 2 (4%) | 1 (2%) | ||
| Body Mass Index (BMI)
n (%) |
Underweight | 1 (2%) | 1 (2%) | 0.751 |
| Normal weight | 10 (20%) | 16 (20%) | ||
| Over weight | 25 (50%) | 21 (42%) | ||
| Obese | 12 (24%) | 10 (20%) | ||
| Very obese | 2 (4%) | 2 (4%) | ||
| Right Ovary (cm3)
Mean ± SD |
Height | 3.98 ± 0.53 | 3.80 ± 0.23 | 0.299 |
| Width | 2.43 ± 0.45 | 2.37 ± 0.31 | 0.4394 | |
| Breadth | 1.88 ± 0.59 | 1.81 ± 0.21 | 0.4312 | |
| Left Ovary
(cm3) Mean ± SD |
Height | 3.82 ± 0.50 | 3.71 ± 0.31 | 0.1892 |
| Width | 2.43 ± 0.46 | 2.34 ± 0.28 | 0.2402 | |
| Breadth | 1.84 ± 0.59 | 1.84 ± 0.25 | 1.000 | |
Furthermore, 60% cases were improved in their menstrual complaints; most common menstrual abnormality improved was Oligomenorrhoea i.e., 71.4% (Table 3).
Table 3: Cases according to improvement in menstrual abnormality after treatment.
| Menstrual Abnormality | Before Treatment (n=50)
n (%) |
No. of Cases Improved
(n=31) n (%) |
% Improvement | p-Value |
| Oligomenorrhoea | 21(42) | 15(48) | 71.4 | 0.06 |
| Amenorrhea | 9(18) | 4(13) | 44.4 | 0.08 |
| Oligomenorrhoea+ Hypomenorrhea | 7(14) | 5(16.1) | 71 | 0.06 |
| Oligomenorrhoea+ Menorrhagia | 13(26) | 7(22.6) | 54 | 0.12 |
In the present study, Myoinositol supplementation 31 out of 50 (66%) cases achieved regular menstrual cycles during therapy. The polycystic ovarian syndrome is a complex and relatively common disorder of women in their reproductive age years, which in most cases is present at the time of birth but manifested only after the menarche/ puberty11-14. Studies have assessed the effectiveness of different agents focusing on the improvement in fertility among PCOS-affected women however; the regulation of menstrual cycle and flow was mostly neglected15,16. This study has highlighted the menstrual irregularities and evaluated the efficacy of a relatively newer therapeutic intervention i.e. Myo-Inositol (MI) in the correction of menstrual irregularities among PCOS-affected women17,18. To the best of the knowledge; this is the first of its kind of study conducted in Pakistan.
Forty-one women (82%) had either low/ minimal (<20 ml) menstrual flow while other 2 (4%) women had heavy (>60 ml) which after treatment was regulated and became normal menstruation flow in 40 (80%) women (p-value = <0.0001). Menstrual cycle duration was also changed from 2-10 days in length (Mean 4.36) to 3-8 days (Mean 4.70) per month. The study results also depicted a remarkable difference in the range of menstrual flow volume which was corrected with Myo-inositol. This proves the efficacy of MI in regulating the menstrual cycles as well as bringing the menstrual volumes to the normal ranges. According to some previous studies more than half (>50%) of all women found significant improvement and normal regulation of menstrual cycle and flow (p-value < 0.001) within three months6,7.
Continuous data of cycle duration, minimum cycle flow and maximum cycle flow were evaluated by applying the students’ t-test and very highly significant differences were found (p-values = <0.0001, <0.0001 and<0.0001) in pre-treatment and post-treatment parameters. Other studies like Genazzani et al., found that the relieving of symptoms, reaching a menstrual cycle regularity and normalization of serum hormone levels were gained positively during the three months regimen of treatment with MI. Ranwa et al. found that 81.8% of cases of oligomenorrhoea with hypomenorrhea had improved menstrual bleeding patterns while 88.67% of oligomenorrhoea cases achieved regular menstrual cycles19. They found that 94.67% of PCOS cases with Oligomenorrhoea (43.66%) followed by Oligomenorrhoea and Menorrhagia (21.13%) Amenorrhea (19.71%) and Hypomenorrhea and Oligomenorrhoea (15.49%) improved to 74.65% and later 77.4-88% who achieved menstrual regularity19. Myo-Inositol therapy administered to patients was more successfully treated in reaching regular menstrual cycles16.
Papaleo et al. found that Myo-Inositol therapy (2gm with folic acid 200 µg administration) for a month resulted in achieving a spontaneous menstrual cycle among 88% of patients which was sustained in 72% of patients during the follow-up period20,21,8. However, the current study had not used folic acid as it was not part of the research. In a larger study conducted by Regidor et al., taking a sample of 2520 had success as 15.1% also achieved conception22.
Investigating the volume of ovaries (a very important marker and critical part of the diagnosis of PCOS); the current study investigated and evaluated the changes brought into the ovarian volume. Mean volume of right ovary decreased from 9.39 cms3 to 7.89 cms3 while the left ovary volume decreased from 8.88 cms3 pre-treatment to 7.91cms3 post-treatment (p-value < 0.001). This supports the effectiveness of Myo-inositol in the regulation of menstrual cycle/ flow.
The study noted the significant mean difference in volumes of all three parameters of both sides of ovaries as in previous studies. Genazzani et al., compared two treatment regimens viz; MI plus folic acid or folic acid alone and found that the earlier group has achieved a significant reduction in ovaries volumes compared to no or little difference in the control group (p-value < 0.05) 18,14. It was noted in the result those ovaries volumes decreased significantly in the treatment group (12.2 ± 0.6 mL to 8.7 ± 0.8 mL, p-value < 0.05) 23. De Cicco et al., Gerli et al. and Genazzani et al. support these findings regarding the correction of menstrual irregularities and achieving ovulation11,24-26.
In the current study mean age was 27.68 ± 4.787 years while a study by La Marca et al., found that the mean age of females was 27.5 ± 6.7 years27. The present study noted that age was an effect modifier of Myo-Inositol therapy efficacy in PCOS in achieving menstrual cycle and flow correction. Accordingly; women below 20 years and 31-40 years of age had better results of correction of menstrual flow severity compared to those of age 21-30 years. BMI is also an effect modifier when evaluating the menstrual flow, the PCOS patients before and after the treatment. It was a small sample interventional study. However, the sampling was consecutive and there was no control arm. Yet the current study has come up with strong findings that Myo-Inositol therapy is very efficacious in treating menstrual irregularities in PCOS women in a local setting.
Polycystic ovary syndrome (PCOS) results because of hyperandrogenic activity of ovaries and there is an increased prevalence of PCOS cases in the Pakistani population. The newer and safer therapies need to be identified and tested in specific populations to generate relevant evidence. The current study in this regard found 2gm/day Myo-Inositol efficacious in the treatment of polycystic ovarian syndrome and correction of menstrual irregularities, menstrual flow, and regulation of volumes of ovaries among a local group of affected women.
The authors would like to thank the hospital for the facilitation and smooth conduction of the research.
The authors declare no conflict of interest.
Ethics approval was granted by the ethics review committee with the reference code: 06911118RKGYN.
Patient consent was taken before the data collection.
The research was a self-funded study.
RK initiated the idea of the study as, a major contributor to data collection and manuscript writing. HS did the literature search and finalization of the article. OK did a major contribution to data collection and literature review. MY contributed to manuscript writing and finalization. MM did the literature search, data analysis and was a major contributor to the finalization of the manuscript draft.
- Piltonen TT, Ruokojärvi M, Karro H, Kujanpää L, Morin-Papunen L, Tapanainen JS, et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS One. 2019;14(12):1-12. doi: 10.1371/journal.pone.0226074
- Unfer V, Nestler JE, Kamenov ZA, Prapas N, Facchinetti F. Effects of inositol (s) in women with PCOS: a systematic review of randomized controlled trials. Int J Endocrinol. 2016;2016:1-12 doi: 10.1155/2016/1849162
- Pkhaladze L, Barbakadze L, Kvashilava N. Myo-inositol in the treatment of teenagers affected by PCOS. Int J Endocrinol. 2016;2016:1-6. doi: 10.1155/2016/1473612
- Sortino MA, Salomone S, Carruba MO, Drago F. Polycystic ovary syndrome: insights into the therapeutic approach with inositols. Front Pharmacol. 2017;8:1-13. doi: 10.3389/fphar.2017.00341
- Unfer V, Facchinetti F, Orrù B, Giordani B, Nestler J. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect. 2017;6(8):647-658. doi: 10.1530/EC-17-0243
- Celentano C, Matarrelli B, Mattei PA, Pavone G, Vitacolonna E, Liberati M. Myo-inositol supplementation to prevent gestational diabetes mellitus. Curr Diab Rep. 2016;16(3):1-7. doi: 10.1007/s11892-016-0726-6
- Nestler JE, Unfer V. Reflections on inositol (s) for PCOS therapy: steps toward success. Gynecol Endocrinol. 2015;31(7):501-505. doi: 10.3109/09513590.2015.1054802
- Heimark D, McAllister J, Larner J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2013: EJ13-0423. Doi: 1507/endocrj.EJ13-0423
- Kamenov Z, Kolarov G, Gateva A, Carlomagno G, Genazzani AD. Ovulation induction with myo-inositol alone and in combination with clomiphene citrate in polycystic ovarian syndrome patients with insulin resistance. Gynecolo Endocrinol. 2015;31(2):131-135. doi: 10.3109/09513590.2014.964640
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012(5):1-187. doi: 10.1002/14651858.CD003053.pub5
- Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014; 6: 1-13. doi: 10.2147/CLEP.S37559
- Harris HR, Titus LJ, Cramer DW, Terry KL. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population‐based case‐control study. Int J Cancer. 2017;140(2):285-291. doi: 10.1002/ijc.30441
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748-758. doi: 10.1093/humupd/dmu012
- Fux Otta C, Wior M, Iraci GS, Kaplan R, Torres D, Gaido MI, et al. Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: a randomized, double-blind, and placebo control trial. Gynecol Endocrinol. 2010;26(3):173-178. Doi: 10.3109/09513590903215581
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. doi: 10.1093/humrep/dey256
- Laganà AS, Vitagliano A, Noventa M, Ambrosini G, D’Anna R. Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: a systematic review and meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2018;298(4):675-684. doi: 10.1007/s00404-018-4861-y
- Unfer V, Porcaro G. Updates on the myo-inositol plus D-chiro-inositol combined therapy in polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2014;7(5):623-631. doi: 10.1586/17512433.2014.925795
- Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24(3):139-144. doi: 10.1080/09513590801893232
- Ranwa M, Nagaria T, Jaiswal J, Arya A. Study of effect of myoinositol on menstrual irregularities and skin problems in polycystic ovarian syndrome cases. Int J Reprod Contracept Obstet Gynecol. 2017;6(6):2310-2318. doi: 10.18203/2320-1770.ijrcog20172120
- Papaleo ED, De Santis L, Baillargeon J, Zacche M, Fusi F, Brigante C, et al. Comparison of myo-inositol plus folic acid vs clomiphene citrate for first line treatment in women with polycystic ovary syndrome. In Proceedings of the 24th Annual Meeting of ESHRE, Barcelona, Spain 2008:6-9.
- Papaleo E, Unfer V, Baillargeon JP, Fusi F, Occhi F, De Santis L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril. 2009;91(5):1750-1754. doi: 10.1016/j.fertnstert.2008.01.088
- Regidor PA, Schindler AE. Myoinositol as a safe and alternative approach in the treatment of infertile PCOS women: a German observational study. Int J Endocrinol. 2016;2016. doi: 10.1155/2016/9537632
- Costantino D, Minozzi G, Minozzi E, Guaraldi C. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: a double-blind trial. Eur Rev Med Pharmacol Sci. 2009;13(2):105-110.
- De Cicco S, Immediata V, Romualdi D, Policola C, Tropea A, Di Florio C, et al. Myoinositol combined with alpha-lipoic acid may improve the clinical and endocrine features of polycystic ovary syndrome through an insulin-independent action. Gynecol Endocrinol. 2017;33(9):698-701. doi: 10.1080/09513590.2017.1313972
- Gerli S, Papaleo E, Ferrari A, Di Renzo GC. Randomized, double blind placebo-controlled trial: effects of myo-inositol on ovarian function and metabolic factors in women with PCOS. Eur Rev Med Pharmacol Sci. 2007;11(5):347-354.
- Genazzani AD, Prati A, Santagni S, Ricchieri F, Chierchia E, Rattighieri E, et al. Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecol Endocrinol. 2012;28(12):969-973. doi: 10.3109/09513590.2012.685205
- La Marca A, Grisendi V, Dondi G, Sighinolfi G, Cianci A. The menstrual cycle regularization following D-chiro-inositol treatment in PCOS women: a retrospective study. Gynecol Endocrinol. 2015;31(1):52-56. doi: 10.3109/09513590.2014.964201
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
