By Shazia Mumtaz1, Hira Salam2, Rubina Gulzar1, Ruqaiya Shahid1
AFFLIATIONS:
- Department of Pathology, Dow International Medical College, Dow University of Health Sciences (DUHS), Karachi, Pakistan.
- Dow Dental Medical College, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-1/008
How to cite: Mumtaz S, Salam H, Gulzar R, Shahid R. Prevalence of Submandibular Gland Metastasis in Squamous Cell Carcinomas of Oral Cavity. Pak J Med Dent. 2022;11(1): 44-49. doi: 10.36283/PJMD11-1/008
Background: The oral squamous cell carcinoma (OSCC), the most common malignant tumor of the oral cavity, have aggressive biological behavior and is treated with surgical excision, lymph node dissection and submandibular gland excision. However, the submandibular gland is rarely involved by OSCC. The study aimed to identify the prevalence of submandibular gland involvement with metastasis in squamous cell carcinoma of oral cavity patients at a tertiary care center in Pakistan.
Methods: The oral cancer patients (139) having wide excisions with neck dissection cases were included from the Dow Diagnostic Research and Reference Laboratory (DDRRL), between May 2017-2019. The cancer cases summary was recorded in a specifically designed protocol. Data was analyzed by SPSS version 21. The Chi-square test was used to find an association between categorical variables and p-value <0.05 was considered statistically significant.
Results: The male to female ratio was 2.7: 1. A wide age range (20-84 years) with a mean age of 46±13 years. Most patients 48 (34.5%) belonged to >51 years of age group. The most frequently involved sites were Buccal mucosa 75 (52.9%), followed by tongue 24 (17.45%). A significant association was recorded between tumor size and tumor thickness (p<0.005), as well as tumor size and histological grade (p=0.023). In this study, only 3 (2.1%) submandibular glands were involved by metastatic carcinoma.
Conclusion: The involvement of the Submandibular gland with metastatic oral squamous cell carcinoma prevalence was very low to warrant efforts towards the preservation of gland in early-stage patients.
Keywords: Oral Squamous Cell Carcinoma; Submandibular Gland; Neck Dissection.
Owing to the momentous impact on prognosis rendered by the nodal spread of oral squamous cell carcinoma, neck dissection has remained an indispensable component of surgical management of oral cancer for over a century4. Routinely dissected cervical lymph node levels in oral cancer patients include levels I–V, but the extent of neck tissue that ought to be surgically removed has remained a subject of debate among clinician scientists5. Radical neck dissection is now largely replaced by more conservative approaches such as supra-omohyoid neck dissection (SOHD) for elective procedures, modified radical neck dissection (MRND), and selective neck dissections (SND)6,7.
Submandibular gland (SMG) is invariably removed as part of neck dissections, including SND because of its location in level Ib notwithstanding the rarity of its involvement by metastases from oral squamous cell carcinomas8. SMGs contribute more than half (70-90%) of unstimulated salivary flow and therefore its removal predisposes patients to xerostomia and its sequelae, a condition that might be worsened if a patient is also subjected to adjuvant radiotherapy8. It is imperative to determine the rationale for SMG removal by delineating the frequency of its involvement with oral cancer metastases. Therefore, the study aimed to identify the prevalence of SMG involvement with metastasis in oral squamous cell carcinoma patients treated with wide excision and neck dissection in the Pakistani targeted population.
METHODS
The oral cancer patients (139) having wide excisions with neck dissection cases were included in this cross-sectional study. The samples were obtained from the histopathological section of the Dow Diagnostic Research and Reference Laboratory (DDRRL), between May 2017-May 2019. Archival data was retrieved following approval from University’s ethical board, from the Histopathology section of Dow Diagnostic Research and Reference Laboratory (DDRRL), Dow University of Health Sciences (DUHS), Pakistan. The institutional review board’s (IRB) reference number is IRB-1206/DUHS/approval/2019.
The inclusion criteria were all oral cancer cases treated with wide local excision with neck dissection including excision of SMG. The cases received without formalin and inconclusive diagnoses were excluded from the study. This study period was from January 2017 until May 2019. The cancer case summary was recorded in a specifically designed protocol. Data was analyzed in SPSS version 21.0. The Chi-square test was used to test the association between categorical variables. Frequency was calculated for age, and gender and tumor sites. p-value <0.05 was considered statistically significant.
The study retrieved n=139 cases of oral squamous cell carcinoma that had undergone wide excision and neck dissection including excision of the submandibular gland from the Histopathology section of Dow Diagnostic Research and Reference Laboratory (DDRRL), Dow University of Health Sciences (DUHS), Pakistan. The male preponderance was recorded with male to female ratio 2.7:1, male 102(73.4%), female 37(26.6%). Overall, the mean age (year) at diagnosis was 46 ± 12(mean ± SD), but when mean age was calculated separately for each gender a statistically significant difference was recorded between males and females with a mean age at diagnosis 8 years older in females compared with males (p-value=0.01). Increasing incidence was noted with increasing age and greatest prevalence recorded in >50 years’ age group (34.5%), followed by 41-50 years 41(29.5%). Slightly more than half of all cases presented with primary tumor located in buccal mucosa 75(54%). The tongue was the second most frequently involved site 24(17.3%) followed closely by lip and lower alveolus/gingiva 12(8.6% /each) as shown in Table 1.
Table 1: Clinicopathological characteristics of the patient.
| Clinicopathological Parameters | Frequency n (%) | ||||||
| Age range | < 20 | 21 – 30 | 31 – 40 | 41 – 50 | >50 | >55 | >60 |
| 1 | 19(13.7%) | 30(21.6%) | 41(29.5%) | 48(34.5%) | – | – | |
| Tumor site | Buccal mucosa | Lip | Tongue | Upper alveolus/ gingiva | Lower alveolus/ gingiva | Palate | Others |
| 75(54.0%) | 12(8.6%) | 24(17.3%) | 4(2.8%) | 12(8.6%) | 2(1.4%) | 10(7.2%) | |
| Tumor thickness/cm | <0.5 | 0.5 – 1 | 1-2 | 2-3 | >3 | p=0.029 | |
| Number of positive lymph nodes (N) | 12 | 41 | 51 | 19 | 9 | p=0.029 | |
In 139 neck dissections received, only three cases were recorded whereby gland was involved with metastatic squamous cell carcinoma, making prevalence in our study population merely 2.1%. We recorded a total of 3 cases exhibiting metastatic disease in submandibular gland, their characteristics are summarized in Table 2.
Table 2 : Characteristics of cases positive for metastatic disease involving the submandibular gland.
| No. | Gender | Age /years | Tumor site | Tumor thickness /cm | Histological grade | PNI | Number of positive LNs | ENE |
| Case 1 | Female | 50 | Lower alveolus | 2.9 | Grade 2 | + | 0 | – |
| Case 2 | Male | 45 | Buccal mucosa | 1 | Grade 2 | + | 3 | + |
| Case 3 | Male | 54 | Buccal mucosa | 1.5 | Grade 2 | – | 2 | – |
PNI- Perineural invasion, LNs- Lymph nodes, ENE- Extra-nodal extension.
A statistically significant correlation was recorded between tumor size (measured as the greatest dimension of tumor on the surface) with tumor thickness (p<0.05) and with histological grade (p=0.023). Tumor thickness was also significantly correlated with a few lymph nodes involved with metastatic disease (p=0.029) (Figures 1 and 2).
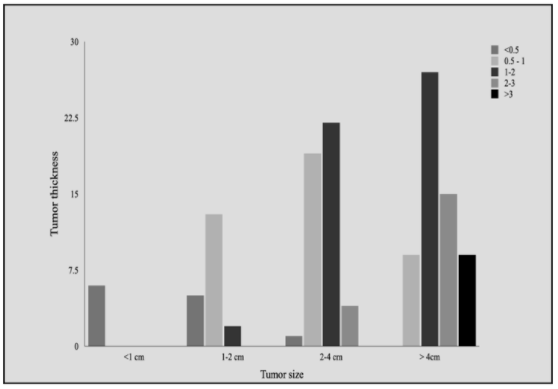
Figure 1: Correlation of tumor size with tumor thickness. With increasing tumor size, tumor thickness also increased with >3cm tumor thickness being most frequently recorded in the >4cm tumor size group (p-value <0.05).
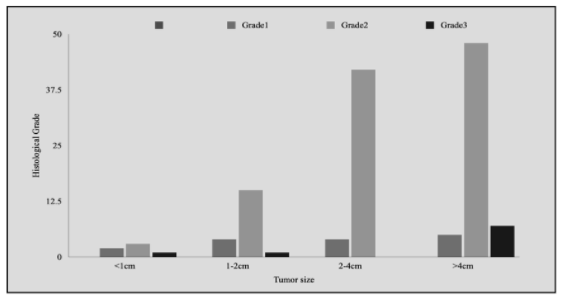
Figure 2: Correlation of tumor size with tumor grade. A positive relationship was recorded between tumor size and histological tumor grade with Grade 3 cases recorded most frequently in the >4cm tumor size group (p-value=0.023).
The paired submandibular glands contribute 70-90% of unstimulated salivary volume9-11. Saliva is crucial for the maintenance of oral health and integrity of dentition with its varied biological functions of aiding speech, mastication and, deglutition also plays a vital role in mediating local immunity under its IgA, lactoferrin, and various enzymes2,10. Resection of SMG, which is routinely performed as a component of neck dissection in oral cancer patients invariably results in reduced salivary flow, hampering salivary function, and xerostomia12. Razfar et al. in their study of long-term clinical outcomes for SMG excision, reported xerostomia in 22.1% of patients13. Another study reported xerostomia in 21% of patients who underwent SMG excision14. In addition to the debilitating effects associated with the reduced salivary flow after excision of the submandibular gland, the question of whether sacrificing SMG routinely with neck dissection even in patients with negative lymph nodes is justified remains a matter of scientific debate4.
In the present study comprising of 139 neck dissections, more than half (54.7%) cases were absent for nodal metastasis and a further 15.8% was involved of a single lymph node. Only 3 cases out of 139 were positive for metastatic disease in the gland making the prevalence of SMG involvement in the present study 2.1%, in concordance with other studies that have also reported low prevalence rates ranging from 0.4%-9.3% 5,12,15. A recently published meta-analysis comprising of 12 studies and 2,126 patients also reported an involvement rate for SMG of 2%, which dropped to 0.1% when involvement by direct spread of tumor was excluded4. In a prospective study conducted, Cetin et al. showed that even in presence of Level Ib metastasis, the rate of SMG gland involvement is rare and can be spared16.
According to a prospective study by Kou et al. male gender, perineural invasion, and proximity of surgical margins to the tumor were prognostic indicators for disease-free survival of stage I and II oral squamous cell carcinoma patients, whereas excision or preservation of SMG had no impact on overall survival of early-stage patients8. Interestingly, 2 out of 3 cases with SMG involvement in the present study also showed perineural invasion. This study also identified male predilection with a higher mean age at presentation in females which is in concordance with other studies17,18. While oral squamous cell carcinoma has reported predilection for tongue in studies from other parts of the world, in the Pakistani population there is a striking predilection for involvement of buccal mucosa in concordance with the present study where buccal mucosa (54%) involvement surpassed tongue (17.3%) involvement significantly17-20. Lymph node involvement is one of the powerful prognostic indicators for metastasis in oral squamous cell carcinoma. In this study tumor thickness significantly, the correlation was seen with lymph node metastasis, a similar finding was also reported in a study by authors Bhurgri et al, Qureshi et al and Ahmed et al 21-23. Perineural and lymphovascular invasion significantly correlate with prognosis24,25.
It was found no significant correlation of perineural invasion, lymphovascular invasion with other histological parameters24. The extranodal extension was found in 22% of cases, similarly, no correlation was established of this parameter with tumor size, tumor grade, and lymph node metastasis25. We report rare involvement of the submandibular gland in oral squamous cell carcinomas i.e., only 3 cases out of 139 cases. All three positive cases had tumor thickness of >1 cm. Salivary gland sparing surgery in oral squamous cell carcinoma can be a new approach with careful selection based on presurgical radiological evaluation of salivary glands and clinical grounds26,27.
The involvement rate of submandibular glands with metastatic oral squamous cell carcinoma was low to warrant efforts towards the preservation of gland and xerostomia and its sequelae in early-stage patients. Radiological evaluation is recommended before patients’ selection for the submandibular sparing. However, further longitudinal cohort studies with a large study population including a comparison of prognostic values of performing submandibular gland sacrificing versus gland-sparing neck dissections are still needed.
The authors would like to acknowledge the Department of Histopathology Dow University of Health Sciences.
The authors declare no conflict of interest.
The study was conducted following approval from University’s ethical board, from the Histopathology section of Dow Diagnostic Research and Reference Laboratory (DDRRL), Dow University of Health Sciences (DUHS), Pakistan. The institutional review board’s (IRB) reference number is IRB-1206/DUHS/approval/2019.
The consent was obtained from the patients of the research study.
SM had analyzed the data and written the manuscript. HA did the literature search, collected the data. RG and RS reviewed the data, analyzed it, and interpreted.
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. doi: 10.1002/ijc.31937
- Panda NK, Patro SK, Bakshi J, Verma RK, Das A, Chatterjee D. Metastasis to submandibular glands in oral cavity cancers: Can we preserve the gland safely? Auris Nasus Larynx. 2015;42(4):322-325. doi: 10.1016/j.anl.2015.02.006
- Malgonde MS, Kumar M. Practicability of submandibular gland in squamous cell carcinomas of oral cavity. Indian J Otolaryngol Head Neck Surg. 2015;67(1):138-140. doi: 10.1007/s12070-014-0803-6
- Yang S, Wang X, Su JZ, Yu GY. Rate of submandibular gland involvement in Oral squamous cell carcinoma. J Oral Maxillofac Surg. 2019;77(5):1000-1008. doi: 10.1016/j.joms.2018.12.011
- Subramaniam N, Balasubramanian D, Reddy R, Rathod P, Murthy S, Vidhyadharan S, et al. Determinants of level Ib involvement in oral squamous cell carcinoma and implications for submandibular gland-sparing neck dissection. Int J Oral Maxillofac Surg. 2018;47(12):1507-1510. doi: 10.1016/j.ijom.2017.11.019
- Bartella AK, Kloss-Brandstätter A, Kamal M, Teichmann J, Modabber A, Hölzle F, et al. “IIb or not IIb”–The necessity of dissection in patients with oral squamous cell carcinoma. J Craniomaxillofac Surg. 2016;44(10):1733-1736. doi: 10.1016/j.jcms.2016.08.003
- Akhtar S. Neck involvement in early carcinoma of tongue. Is elective neck dissection warranted? J Pak Med Assoc. 2007;57(6):305-307.
- Kou Y, Zhao T, Huang S, Liu J, Duan W, Wang Y, et al. Cervical level IIb metastases in squamous cell carcinoma of the oral cavity: a systematic review and meta-analysis. Onco Targets Ther. 2017; 10: 4475-4483. doi: 10.2147/OTT.S143392
- Chen TC, Lou PJ, Ko JY, Yang TL, Lo WC, Hu YL, et al. Feasibility of preservation of the submandibular gland during neck dissection in patients with early-stage oral cancer. Ann Surg Oncol. 2011;18(2):497-504. doi: 10.1245/s10434-010-1294-7
- Yu Y, Daly ME, Farwell DG, Luu Q, Gandour‐Edwards R, Donald PJ, et al. Level IB nodal involvement in oropharyngeal carcinoma: Implications for submandibular gland‐sparing intensity‐modulated radiotherapy. Laryngoscope. 2015;125(3):608-614. doi: 10.1002/lary.24907
- Naidu TK, Naidoo SK, Ramdial PK. Oral cavity squamous cell carcinoma metastasis to the submandibular gland. J Laryngol Otol. 2012;126(3):279-284. doi: 10.1017/S0022215111002660
- Cunning DM, Lipke N, Wax MK. Significance of unilateral submandibular gland excision on salivary flow in noncancer patients. Laryngoscope. 1998;108(6):812-815. doi: 10.1097/00005537-199806000-00007
- Razfar A, Walvekar RR, Melkane A, Johnson JT, Myers EN. Incidence and patterns of regional metastasis in early oral squamous cell cancers: feasibility of submandibular gland preservation. Head Neck. 2009;31(12):1619-1623. doi: 10.1002/hed.21129
- Springborg LK, Møller MN. Submandibular gland excision: long-term clinical outcome in 139 patients operated in a single institution. Eur Arch Otorhinolaryngol. 2013;270(4):1441-1446. doi: 10.1007/s00405-012-2175-4
- Jaguar GC, Lima EN, Kowalski LP, Pellizon AC, Carvalho AL, Alves FA. Impact of submandibular gland excision on salivary gland function in head and neck cancer patients. Oral Oncol. 2010;46(5):349-354. doi: 10.1016/j.oraloncology.2009.11.018
- Cetin AC, Dogan E, Ozay H, Kumus O, Erdag TK, Karabay N, et al. Submandibular gland invasion and feasibility of gland-sparing neck dissection in oral cavity carcinoma. J Laryngol Otol. 2018;132(5):446-451. doi: 10.1017/S0022215118000592
- Malik A, Joshi P, Mishra A, Garg A, Mair M, Chakrabarti S, et al. Prospective study of the pattern of lymphatic metastasis in relation to the submandibular gland in patients with carcinoma of the oral cavity. Head Neck. 2016;38(11):1703-1707. doi: 10.1002/hed.24508
- Dhanuthai K, Rojanawatsirivej S, Thosaporn W, Kintarak S, Subarnbhesaj A, Darling M, et al. Oral cancer: A multicenter study. Med Oral Patol Oral Cir Bucal. 2018; 23(1): 23-29. doi: 10.4317/medoral.21999.
- Rai HC, Ahmed J. Clinicopathological correlation study of oral squamous cell carcinoma in a local Indian population. Asian Pac J Cancer Prev. 2016;17(3):1251-1254. doi: 10.7314/APJCP.2016.17.3.1251
- Azhar N, Sohail M, Ahmad F, Fareeha S, Jamil S, Mughal N, et al. Risk factors of Oral cancer-A hospital based case control study. J Clin Exp Dent. 2018; 10(4): 396-401. doi: 10.4317/jced.54618
- Bhurgri Y. Cancer of the oral cavity-trends in Karachi South (1995-2002). Asian Pac J Cancer Prev. 2005;6(1):22-26.
- Qureshi MA, Syed SA, Sharafat S. Lip and oral cavity cancers (C00-C06) from a mega city of Pakistan: Ten-year data from the Dow Cancer Registry. J Taibah Univ Med Sci. 2021;16(4):624-627. doi: 10.1016/j.jtumed.2021.02.001
- Ahmed SQ, Junaid M, Awan S, Choudhary MM, Kazi M, Masoom A, et al. Relationship of tumor thickness with neck node metastasis in buccal squamous cell carcinoma: an experience at a tertiary care hospital. Int Arch Otorhinolaryngol. 2017;21:265-269. doi: 10.1055/s-0037-1599061
- Mücke T, Kanatas A, Ritschl LM, Koerdt S, Tannapfel A, Wolff KD, et al. Tumor thickness and risk of lymph node metastasis in patients with squamous cell carcinoma of the tongue. Oral Oncol. 2016;53:80-84. doi: 10.1016/j.oraloncology.2015.11.010
- Haksever M, Inancli HM, Tunçel Ü, Kürkçüoğlu ŞS, Uyar M, Genç Ö, et al. The effects of tumor size, degree of differentiation, and depth of invasion on the risk of neck node metastasis in squamous cell carcinoma of the oral cavity. Ear Nose Throat J. 2012;91(3):130-135.
- Jardim JF, Francisco AL, Gondak R, Damascena A, Kowalski LP. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44(1):23-28. doi: 10.1016/j.ijom.2014.10.006
- Matsushita Y, Yanamoto S, Takahashi H, Yamada SI, Naruse T, Sakamoto Y, et al. A clinicopathological study of perineural invasion and vascular invasion in oral tongue squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44(5):543-548. doi: 10.1016/j.ijom.2015.01.018
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
