By Madiha Soban1, Iftikhar Ahmed2, Navaid Siddiqui3, Faisal Ahmed4, Asher Fawwad5, Tahira Assad6
AFFLIATIONS:
- Department of Biochemistry, Karachi Institute of Medical Sciences, Karachi, Pakistan.
- Department of Basic Sciences, Baqai Medical University, Karachi, Pakistan.
- Department of Medicine, Bahria University Medical and Dental College, Karachi, Pakistan.
- Department of Family Medicine, Baqai Medical University, Karachi, Pakistan.
- Department of Biochemistry, Baqai Medical University, Karachi, Pakistan.
- Department of Pharmacology, Karachi Institute of Medical Sciences, Karachi, Pakistan.
Background: Glutathione (GSH) used for skin lightening can also affect Glucose metabolism in diabetics because of its antioxidant properties. This study aimed to determine the effects of systemic glutathione on various parameters in type 2 diabetes.
Methods: It was an interventional study (open label trial) conducted at Skin care clinic and Baqai Institute of Diabetes and Endocrinology from August 2019 to January 2020. Total patients included in this study were 46 who met the inclusion criteria along with 20 participants as controls. Oral Glutathione (500 mg B.D) was given to both groups for 18 weeks. Pre and post treatment Plasma Glutathione, fasting blood sugar (FBS), random blood sugar (RBS), and HbA1c levels were evaluated. Taylor hyperpigmentation scale was used to measure skin tone. BMI and Anthropometric measurements were also measured. Paired t-test/dependent t-test and Chi squared test was performed and p < 0.05 was considered statistically significant.
Results: Oral Glutathione caused significant reduction in FBS, RBS and HbA1c levels in type 2 diabetics. Mean values of both random and fasting blood sugar significantly decreased in patients group. RBS decreased from 232±10.04 mg/dl to 178±10.37 mg/dl (<0.001*), whereas FBS decreased from 136±10.42mg/dl to 129.24±102.78 mg/dl (<0.002*). HbA1c was significantly decreased in patients group from 7.72 ± 2.04 to 6.66 ± 1.37 (<0.001). Weight, BMI, and systolic blood sugar were also decreased in both groups p=< 0.05, <0.05, 0.005 respectively.
Conclusion: Systemic glutathione used for skin tone lightening in diabetics caused significant reduction in FBS, RBS and HBA1c levels. Glutathione can be used as an adjuvant therapy in diabetics.
Keywords: Diabetes Mellitus; Glutathione; Skin Pigmentation.
The major health problem in 21st century will be the epidemic of diabetes, particularly in underdeveloped countries1. It was estimated that these countries would have an occurrence of diabetes which will rise to 67% from the year 2010 to 2030. Worldwide, the prevalence of diabetes mellitus was found to be 8.5% in the year 2014, which caused a number of deaths2. Increased levels of glucose in the blood for a long period, lead to both microvascular and macrovascular complications3.
During prolonged hyperglycemia, the activity of Polyol pathway is found to be increased. The frequency of Skin diseases is around 30% in diabetics4. Few of these cutaneous problems are linked with resistance to insulin, which appears even before the patient is labeled to have type 2 diabetes5. The assessment of skin problems in pre-diabetic and type 2 diabetic patients is of enormous significance as it may prevent the secondary infections and complications of diabetes.
There is a wide range of antioxidants in the body that try to prevent any damage by free radicals6. One of the important among these is reduced Glutathione that is produced by virtually all cells in the body7. Glutathione is a naturally occurring low molecular weight tri-peptide that is made up of the following amino acids: glutamate, cysteine, and glycine in two steps reactions, which are catalyzed by γ-l-glutamyl-l-cysteine: glycine ligase and glutathione synthetase8. Glutathione controls redox balance inside the cells. After the discovery of its antimelanogenic properties, glutathione was used to lighten the colour of skin9. It works by inhibiting the enzyme tyrosinase and by scavenging highly reactive free radicals. It tilts the process of melanogenesis from darker eumelanin to light phaeomelanin10.
The production of reactive oxygen species and free radicals increases during high blood glucose levels however, during normal blood glucose levels oxidative stress is minimum11. Chronic increase in blood glucose levels also causes a decrease in antioxidant levels in the body including Glutathione, which can increase oxidative damage12. There are still no meticulous studies in the world that show how oral or intravenous Glutathione that is used for skin lightening or hyper-pigmentation, can also have effects on glucose metabolism in type 2 diabetics or metabolic syndrome patients. The present study aimed to determine the effects of systemic Glutathione used for skin tone lightening and pigmentation on blood glucose levels in diabetics.
This interventional (open label trial) study was carried out at Baqai Institute of Diabetology and Endocrinology (BIDE) and Skin Care Clinic, Karachi after approval from Ethics and Board of Advanced Studies and Research (BASR), Baqai Medical University (ERC no: BMU/EC/07-2019-03). Total 66 participants of either gender, aged between 20 to 50 years, were included in this study. Twenty participants who did not have any disease were taken as controls (Group B). 46 patients who visited to the outpatient clinic for skin problems along with type 2 diabetes All Participants were given Systemic Glutathione (Tablet Precos® from CosDerm Skin Care, Pakistan) as per the clinical requirement specified in standard guidelines for skin tone lightening / pigmentation (L-Glutathione 500mg + vitamin C 60mg).
Vitamin C is added to increase the absorption of Glutathione and it affects HbA1c levels only at the dose 1000mg /day ) B.D for 18 weeks, i.e.: 126 days via the oral route. The skin changes start to appear after 10 weeks of glutathione therapy. Pre and post treatment Glutathione, FBS, RBS and HbA1c levels were assessed. The beginning day of treatment was counted as day zero (base line). After overnight fasting, venous blood samples (10 mL) were drawn in vacutainers from the forearm vein of all participants. Blood samples were then added to the gel tube for thirty minutes. The gel tube was centrifuged at 2000 RPM for 10 minutes to separate the Serum. Physiological parameters (Systolic and Diastolic blood pressure), as well as anthropometric measurements (Weight and Height), were also measured by standard techniques both at base line and at the end of treatment. BMI was calculated as body weight (kg) divided by the square of the measured height (m2).
Taylor’s hyperpigmentation scale was used to measure the skin tone. It is a new visual scale developed to provide an inexpensive and convenient method to assess skin color and monitor the improvement of hyperpigmentation following therapy. Two body sites, which were non-exposed to the sun, were measured with Taylor hyperpigmentation cards. The effectiveness and side effects were assessed after 18 weeks (126 days) on cessation of treatment. The lab work was done at BIDE and Biochemistry Laboratory, Department of Biochemistry, Baqai University, Karachi. The data of participants, their anthropometric measurements, baseline tests and biochemical parameters was assembled categorically on Microsoft Office Excel 2007. Results were compared based on changes in biochemical parameters and a p value less than 0.05 was considered to be statistically significant. Patients to whom glutathione was given were categorized as Group A (Patients Group) while the persons without any disease to whom glutathione was given were considered as controls and were categorized as Group B.
All physical and biochemical parameters were compared from day zero (start of treatment) until 18th week, 126 days (end of treatment) for Group A and Group B respectively. A non-Probability convenience sampling technique was used. The sample size was calculated by online site: https://select-statistics.co.uk/; the Confidence level was taken as 95%, power was kept 80%, the hypothesized difference was found to be 3.5, Population variance was 16 and the sample size calculated was 2113. Statistical analysis was done by using SPSS version 23.0. To know the significance comparison of different variables parametric and non-parametric tests (Unpaired t-test / Independent t-test, Paired t-test / dependent t-test and Chi squared) were performed and p-value < 0.05 was considered statistically significant.
The mean values of baseline physiological and anthropometric parameters between Group A and Group B at day 126. It was observed that administration of glutathione caused a significant reduction in weight (p< 0.05), BMI (p< 0.05) and systolic blood pressure (p<0.05) in both the group’s A and B. Diastolic blood pressure was decreased insignificantly only in diabetics (Table 1).
Table 1: Baseline physiological and anthropometric parameters.
| Parameters | Group A
(day 0) |
Group B
(day 0) |
Group A
(day 126) |
Group B
(day 126) |
p-Value |
| Age (years) | 43.67 ± 11.60 | 38.80 ± 9.11 | 43.67 ± 11.60 | 38.80 ± 9.11 | 0.101 |
| Weight (kg) | 82.00 ± 12.35 | 68.20 ± 10.50 | 80.5 ± 11.35* | 67.0 ± 10.10* | < 0.05 |
| Height (m) | 167.28 ± 11.36 | 176.45 ± 12.85 | 167.28 ± 11.36 | 176.45 ± 12.85 | 0.005 |
| BMI (kg/m2) | 29.26 ± 4.28 | 21.90 ± 1.65 | 28.16 ± 3.28* | 20.40 ± 1.55* | < 0.05 |
| SBP (mm Hg) | 150±10 | 120±10 | 140±10* | 110±10* | <0.05 |
| DBP (mm Hg) | 100±10 | 70±20 | 90±10 | 70±20 | 0.005 |
Group A: Patients group (n=46), Group B: Control group (n=20), BMI: Body Mass Index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, *indicates p-value statistically significant, Test applied unpaired t-test / Independent t-test.
While comparing the mean values of RBS and FBS between Group A and Group B at day 126; it was seen that after glutathione intervention mean values of both the random and fasting blood sugar decreased significantly in Group A. RBS decreased from 232±10.04 mg/dl to 178±10.37 mg/dl (p<0.001*). Whereas, FBS was decreasing from 136±10.42mg/dl to 129.24±102.78 mg/dl (p<0.002*) in Group A after glutathione intervention as shown in Table 2.
Table 2: Effect on random and fasting blood sugar.
| Parameters | Group | Day 0
Mean±SD mg/dl |
Day 126
Mean±SD mg/dl |
Mean
Difference mg/dl |
p-Value
|
| Random Blood Sugar (RBS) | A | 232 ± 10.04 | 178 ± 10.37* | 54 ± 9.67 | <0.001 |
| B | 145 ± 10.35 | 143 ± 11.35 | 02 ± 1.00 | 0.005 | |
| Fasting Blood Sugar
(FBS) |
A | 136 ± 10.42 | 129.24 ±102.78* | 07.76 ± 2.79 | <0.002 |
| B | 80.94 ± 10.7 | 80.62 ± 2.71 | 0.32 ± 8.09 | 0.018 |
Group A: Patients group (n=46), Group B: Control group (n=20), RBS: Random Blood Sugar, FBS: Fasting Blood Sugar, *indicates p-value statistically significant, Test applied: Unpaired t-test / Independent t-test.
While comparing the mean difference of biochemical parameters between Group A and Group B at day 126 after Glutathione intervention, it was observed that HbA1c was significantly decreased in Group A from 7.72±2.04 to 6.66±1.37. Whereas, the level of glutathione increased from 5.67±1.42 μmol/L to 10.24±2.78 μmol/L in Group A and from 5.56±1.76 μmol/L to 10.62±2.71 μmol/L in Group B after the glutathione intervention (Table 3).
Table 3: Effect on biochemical parameters glycated hemoglobin (HbA1c) and Glutathione.
| Parameters | Group | Day 0
Mean±SD |
Day 126
Mean±SD |
Mean
Difference |
p-Value |
| HbA1c (%) | A | 7.72 ± 2.04 | 6.66 ± 1.37* | 1.058 ± 1.17 | < 0.001 |
| B | 4.97 ± 0.35 | 4.87 ± 0.35 | 0.10 ± .51 | 0.214 | |
| Glutathione (μmol/L) | A | 5.67 ± 1.42 | 10.24 ± 2.78* | – 4.57 ± 2.79 | 0.535 |
| B | 5.56 ± 1.76 | 10.62 ± 2.71* | – 5.06 ± 3.09 | 0.015 |
Group A: Patients group (n=46), Group B: Control group (n=20)* indicates p value statistically significant, Test applied: Unpaired t-test / Independent t-test.
The present study demonstrated that glutathione when given in the dose of 500 mg B.D for 18 weeks orally, showed skin lightening effects in both the groups at around 14th to 16th week, which was evident in various regions of the body. This finding was confirmed by statistically significant higher levels of glutathione in serum of both Group A (Patients group) and Group B (control group) participants as compared to their pre-treatment serum Glutathione levels which were found to be low. Skin color improvement was found to be 87% amongst group A (patients) and 85% in Group B (control) (Figure 1).
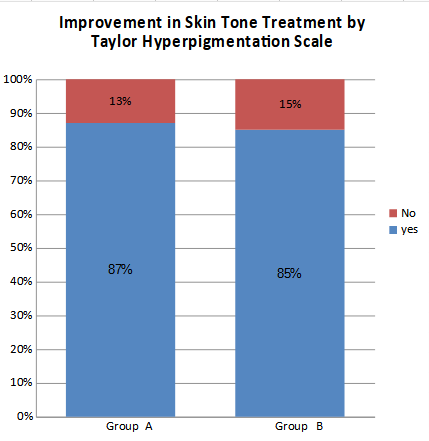
Figure 1: Effect on skin tone in patients after Glutathione intervention.
In the current study, it was found that the intervention of oral glutathione in the dose of 500mg BD for 18 weeks for skin tone lightening in type 2 diabetics caused significant reduction in random and fasting blood sugar levels and HBA1c. Weight, BMI and systolic blood pressure were decreased in both the control and patients group. Tissue damage in type 2 diabetes includes abnormal signaling through protein kinase C, elevated advanced glycation end products, and the alteration in the aldose reductase pathway. The present study suggested that increasing glutathione levels with oral supplementation is a vital intervention to decrease diabetic oxidative stress directly and it could provide an innovative and inexpensive form of nutritional treatment. When used as an adjunct to standard glycemic management, this approach could substantially reduce tissue damage14.
Glutathione is a potent antioxidant that is naturally present in human cells and scavenges the free radical species, thus improving the body’s immunity as well as detoxifying the harmful oxidants. Its function also includes lightening of skin tone by deactivation of tyrosinase enzyme that causes the conversion of melanin to a lighter color pigment15. In the current study, the patients experienced mild to moderate adverse effects ranging from minor dizziness (12%), diarrhea (6%), vomiting (5%), deranged liver functions (1%) after the intervention of Glutathione. None of the patients experienced anaphylactic shock or any other life-threatening side effect because the drug used through oral, buccal or cutaneous route is relatively safe as compared to intravenous route16.
The present study demonstrated that glutathione when given in the dose of 500mg B.D for 18 weeks orally, showed skin lightening effects in both the groups at around 14th to 16th week, which was evident in various regions of the body. This finding was confirmed by statistically significant higher levels of glutathione in serum of both Group A (Patients group) and Group B (control group) participants as compared to their pre-treatment serum Glutathione levels which were found low. Skin color improvement was found to be 87% amongst group A (patients) and 85% in Group B (control).
A similar study was conducted in Philippines in which Glutathione Lozenges were given to thirty females with either skin type IV or type V for eight weeks daily, the researchers saw a significant decline in the Melanin indices along with no severe clinical adverse effects in the participants15. The study also indicated a statistically significant effect of oral glutathione on blood sugar levels. It was found that Glutathione intervention for 126 days caused a reduction in FBS, RBS and HbA1c levels in diabetics.
Various researches have indicated that Diabetes Mellitus leads to mutilation in the antioxidant capability of the patients17,18. Low levels of Glutathione were found in patients with Diabetes as compared to participants with Impaired Glucose Tolerance (IGT) and normal subjects. When comparison was made between the participants with impaired glucose metabolism and normal subjects, it was found that lower glutathione levels were present in the participants with IGT19. This indicates that low levels of glutathione might be associated with the pathogenesis of diabetes mellitus.
Our finding was also supported by the research that showed decreased levels of glutathione and the increased levels of redox markers like Catalase activity in Diabetes Mellitus type 2 patients when they were compared to the controls20. Glutathione (GSH) is an abundant endogenous antioxidant synthesized in large quantities in the liver. It is transported via blood flow to tissues when there is an imbalance between the production of reactive oxygen species and the endogenous antioxidant mechanisms21. Extracellular GSH is not able to pass through cell membranes; however, following enzymatic breakdown into its constituent amino acids, these amino acids can traverse the membrane. Once inside the cell, the amino acids are available for the re-synthesis of GSH. GSH is utilized irreversibly when its product of oxidation Glutathione disulfide (GSSG) is not recycled into GSH by glutathione reductase22.
In another study, reduced levels of glutathione were found in vascular straight muscles along with oxidative stress that was caused by an increased level of blood sugar/hyperglycemia23. Another study suggested a negative relationship between the Glutathione level and HbA1c values in diabetes mellitus patients24. Some reports have shown that GSH production gradually decreases in patients with diabetes with complications and that this decrease is dependent on the degree of hyperglycemia25. Previously, in a study, it was found that increased production of free radicals causes a decline in defense mechanisms of cellular antioxidants that give rise to systemic insulin resistance and lipid peroxidation leading to cellular damage26.
The intervention of systemic glutathione used for skin tone lightening in diabetics caused a significant reduction in random and fasting blood sugar levels and HBA1C. Weight, BMI and systolic blood sugar were also decreased in both groups. Glutathione can be used as adjuvant therapy in diabetics for the improvement in FBS, RBS and HBA1c levels.
The authors are thankful to the staff of Baqai Institute of Diabetology and Endocrinology, for technical support.
There is no conflict of interest to declare.
ERC was taken from the ethical Committee, Baqai University (ERC no: BMU/EC/07-2019-03).
Grant was received from Baqai Medical University.
A written and informed consent was taken before the study.
MS did a literature survey, data collection and manuscript writing. IA conceptualized, supervised the project and finally approved the manuscript. NS helped in data collection and interpretation. FA did the statistical analysis and editing of manuscript and AF helped in data collection and editing. TA did bibliography, final editing of manuscript and submission of the paper. All authors approved the final version of the manuscript.
- Jaacks LM, Siegel KR, Gujral UP, Narayan KV. Type 2 diabetes: A 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30(3):331-343.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88-98.
- Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539-1549.
- Bustan RS, Wasim D, Yderstraede KB, Bygum A. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state – a systematic review. Dan Med J. 2017;64:1-10.
- González-Saldivar G. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther. 2017;7:37-51.
- Saif-Elnasr M, Ibrahim IM, Alkady MM. Role of Vitamin D on glycemic control and oxidative stress in type 2 diabetes mellitus. J Res Med Sci. 2017; 22: 22.
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11-26.
- Sonthalia S, Daulatabad D, Sarkar R. Glutathione as a skin whitening agent: facts, myths, evidence and controversies. Indian J Dermatol Venereol Leprol. 2016;82:262-272.
- Weschawalit S, Thongthip S, Phutrakool P, Asawanonda P. Glutathione and its antiaging and antimelanogenic effects. Clin Cosmet Investig Dermatol. 2017; 10: 147-153.
- Mohiuddin AK. Skin lightening and management of hyperpigmentation. Pharma Sci Anal Res J. 2019;2(2):1-66.
- Ahmadinejad F, Geir Møller S, Hashemzadeh-Chaleshtori M, Bidkhori G, Jami MS. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants. 2017;6(3):1-15.
- Nair A, Nair BJ. Comparative analysis of the oxidative stress and antioxidant status in type II diabetics and nondiabetics: A biochemical study. J Oral Maxillofac Pathol. 2017; 21(3): 394-401.
- Gunawardena HP, Silva R, Sivakanesan R, Ranasinghe P, Katulanda P. Poor glycaemic control is associated with increased lipid peroxidation and glutathione peroxidase activity in type 2 diabetes patients. Oxidative medicine and cellular longevity. 2019;2019:1-10.
- Moldogazieva NT, Mokhosoev IM, Mel’nikova TI, Porozov YB, Terentiev AA. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxidative medicine and cellular longevity. 2019;2019:1-14.
- Handog EB, Datuin MS, Singzon IA. An open‐label, single‐arm trial of the safety and efficacy of a novel preparation of glutathione as a skin‐lightening agent in Filipino women. Int J Dermatol. 2016;55(2):153-157.
- Enns GM, Cowan TM. Glutathione as a redox biomarker in mitochondrial disease—Implications for therapy. J Clin Med. 2017;6(5):50:1-17.
- Paschalis V, Theodorou AA, Margaritelis NV, Kyparos A, Nikolaidis MG. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic Biol Med. 2018;115:288-297.
- Zych M, Wojnar W, Borymski S, Szałabska K, Bramora P, Kaczmarczyk-Sedlak I. Effect of rosmarinic acid and sinapic acid on oxidative stress parameters in the cardiac tissue and serum of type 2 diabetic female rats. Antioxidants. 2019;8(12):1-22.
- Kalkan IH, Suher M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak J Med Sci. 2013;29(4):938-942.
- Spanidis Y, Mpesios A, Stagos D, Goutzourelas N, Bar‑Or D, Karapetsa M, et al. Assessment of the redox status in patients with metabolic syndrome and type 2 diabetes reveals great variations. Exp Ther Med. 2016;11(3):895-903.
- Bissinger R, Bhuyan AA, Qadri SM, Lang F. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2019;286(5):826-854.
- Gould RL, Pazdro R. Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients. 2019;11(5):1-21.
- Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus–A comprehensive review. J Diabetes Complications. 2020;34(8):1-47.
- Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13(2):1165-1172.
- Gawlik K, Naskalski JW, Fedak D, Pawlica-Gosiewska D, Grudzień U, Dumnicka P, et al. Markers of antioxidant defense in patients with type 2 diabetes. Oxid Med Cell Longev. 2016;2016:1-6.
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816-823.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
