By Mahrukh Sheikh1, Marium Zaheer1, Syeda Sadia Zafar1, Saba Hassan Shamim2, Arsala Urooj1, Lubna Avesi2
AFFLIATIONS:
- Department of Oral Pathology, Dr. Ishrat Ul Ebad Khan institute of Oral Health Sciences, DOW University of Health Sciences (DUHS), Karachi, Pakistan.
- Department of Pathology, Dow International Medical Colleges, DOW University of Health Sciences (DUHS), Karachi, Pakistan.
Background: The Covid-19 pandemic effected individuals worldwide. A significant number of specimens examined by pathologists are obtained from oropharyngeal region containing a highly viral titer along with increased preference of the virus to these tissues compared to other areas of the body requiring. The need of knowledge, attitudes such as appropriate Personal Protective Equipment (PPE) along with discarding of samples and adherence to protocols while dealing with these samples is necessary. Therefore, we aimed to assess the knowledge, attitude and practices of oral pathologists pertaining to modifications in laboratory protocols issued by various international organizations.
Methods: A validated questionnaire with 22 close-ended questions was given to academic faculty, practicing pathologists and trainees of the pathology department working in a government institute (n=37). Statistics were applied and p<0.05 was considered statistically significant.
Results: In this study 16 (73.7%) of the individuals were found to be well aware of the possible modifications in protocols. In addition, 12 (50%) pathologists recommended the use of complete PPE while performing various procedures. Furthermore, 15 (68.2%) individuals were satisfied with their laboratory design and training to combat the possible risks of the disease. p value was found to be 0.50, which shows an insignificant association between pathologists and laboratory practices.
Conclusion: The results of the study can be considered satisfactory as they indicate that 16(73.7%) pathologists dealing with oropharyngeal specimens are aware of the modified guidelines regarding laboratory protocols, handling of specimens, architecture and personal protective equipment provided by organizations such as Center for Disease Control and World Health Organization.
Keywords: Covid-19 Pandemic; Pathologists; Laboratory.
The implications of Covid-19 pandemic have been everywhere. With the current second wave of the disease approaching in Pakistan, it is expected to become more lethal with severe consequences. Despite the fact that the pandemic has affected the entire population, health care professionals, including dentists have been one of the sectors primarily afflicted1. While it has been established that the disease poses a serious threat to health care providers, there are certain avenues still left rather unexplored such as whether the virus can be a health hazard for pathologists in contact with oral specimens working in the histopathology and hematology laboratories.
The major route of transmission of the virus has been human to human transmission in the form of air borne droplets, in addition to this it can also be carried through indirect routes such as contact with contaminated objects2. After contact with an infected individual or less likely a contaminated surface the virus can remain viable for an average of 5 days and in some cases up to 24 days3. Most of the individuals contract the disease in a period of 12.5 days. Initial symptoms include fever, respiratory symptoms such as dyspnea and muscle soreness in majority of people4. Less frequently encountered symptoms include sore throat, diarrhea and headache5. Laboratory findings of the disease although nonspecific may include leukocytosis, increased liver and muscle enzymes. Image findings are similar to that pneumonia but with a more peripheral distribution and presence of opacities6. The virus has increased affinity for respiratory tissues, therefore it can be isolated from saliva, nasopharynx and lower respiratory tract specimens confirming the fact that biopsies and specimens obtained from oropharyngeal regions used for diagnosis of oral premalignant and malignant conditions can also serve as a possible route of transmission resulting in an increased risk of disease among oral pathologists7–9.
The pandemic has created a state of unrest among the entire population including pathologists because of the virus is unknown and unpredictable. Pathologists need to be highly attentive regarding the steps performed in processing of oropharyngeal specimens which can serve as a potential reservoir of the virus10. Necessary precautions must be taken to reduce the spread of disease from the laboratory and individuals working in it such as reduction of outpatient visits and admissions for elective surgeries. Allowing of laboratory personnel to work in shifts and staggered meal breaks to avoid close contact with other persons. In order to reduce the potential threat of spread of the virus from specimens, there can be reduction of aerosol producing steps in processing of specimens, establishing a chain of command along with emergency and contingency planning11.
A number of studies have been performed to assess the knowledge, attitude and practices Pakistani population regarding Covid-19 12, 13. Since the diagnostic facilities are being provided with pathologist working round the clock, it is imperative to know whether oral pathologists are aware and have made the necessary changes in their practices as recommended by World Health Organization (WHO) and Centre for Disease Control (CDC). The aim of this study was to evaluate the knowledge, attitude and practices of pathologists dealing with oropharyngeal specimens serving at a public and private setup in Karachi.
A validated questionnaire obtained after taking consent from authors comprising of questions regarding modified knowledge, attitude and practices of oral pathologists in wake of Covid-19 pandemic was distributed to consultant oral pathologists and individuals enrolled in a post graduate program in the Department of Pathology14. The study was conducted in the year 2020 for duration of one month from October 2020 to November 2020. The department of Oral Pathology, Dow University of Health Sciences, provided ethics approval. A soft copy was also constructed using Google forms. Consent form was also attached with the questionnaire. Based on inclusion criteria which was first hand dealing of oropharyngeal specimens any participant was excluded if he or she was absent from duty due to any reason. Any technical staff also dealing with specimens was excluded. Similarly, incomplete forms were excluded from the final analysis. Sample size was not collected because of the limited number of pathologists. A total of 37 individuals were approached out of which 30 responded and all were eligible for the study.
There were 15 histopathologists, 15 cytopathologists while 7 were hematologists. The questionnaire consisted of 22 close-ended questions highlighting whether individuals are aware of modified laboratory protocols. The procedure presents the highest risk of transmission of virus, regarding the use of Personal protective equipment (PPEs) to assess whether individuals suggested the use of a complete PPE including N-95 masks, Head cap, Gloves, protective eye wear, Gown and shoe covers). Alternatively, selective PPE consisting of N-95 masks, Head cap and gloves only or N-95 masks, Head cap and Gloves + Protective eyewear or N-95 masks, Head cap and Gloves + Protective eyewear + Gown. Another question measured the attitude of improper discarding of different specimens including blood/plasma, aspirate and histopathology chemicals and their possible role in increasing rate of transmission of virus and whether accurate monitoring of labs is being performed according to given standards. Statistical analysis was done using SPSS version 21.0. Frequencies and percentages were calculated and reported for each response since it is a descriptive study and p values were calculated where applicable.
A total number of 30 individuals responded to the questionnaire out of which 25 were females and 5 males with age ranging between 24 and 46 years and a mean age of 37.31 years. Out of all the respondents, the highest proportion was of practicing pathologists with ten individuals responding in this group followed by eight persons from the academic faculty and four postgraduate trainees. Sixteen individuals belonged to a government institute while five were part of a semi government organization and one was part of a private hospital.
All of the participants in the study urged the need for additional safety measures in routine laboratory procedures in lieu of the pandemic, twelve individuals indicated the need for improvement in layout of laboratory, equipment, containment of samples transported in laboratory along with modifications in request form and number of personnel working in the laboratory attire and decontamination of surfaces. Eight individuals were found to be a strong advocate of switching to better and more effective PPEs. Five emphasized the need of decontamination of surfaces, equal number of people that is four were concerned about containment of samples transported and change in methods of recording data. Three participants were in favor of better laboratory layout and individuals highlighting the importance of laboratory equipment and number of personals were two in each group.
Majority of the participants, 16(73.7%) were aware of modified guidelines for performing laboratory procedures provided by WHO and CDC. While 6(26.3%) did not have any knowledge of the sort. Regarding possible type of specimens, this can serve as a source of transmission of virus, 17 (77.3%) personals including 8 practicing pathologists. In addition, 7 people of the academic faculty and 2 trainees believed formalin fixed specimens to have the lowest risk of transmission of the virus, followed by cytological smears suggested by 2(9.1%) and 3(13.5%) considered aspirates, blood and plasma of having increased risk of transmission (Figure 1).
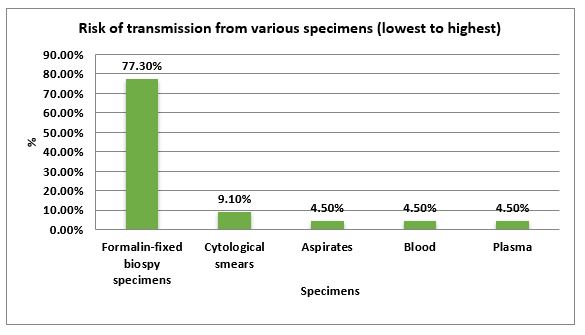
Figure 1: Risk of transmission of virus from various specimen types.
A total of 18(85.7%) participants were of the opinion that specimens obtained such as oral microbial smears, those obtained from periodontal pockets and aspirations of oral lesions constitute a high-risk virus being carried while 2(5.3%) individuals suggested oral specimens possess a moderate risk. While, 1(4.5%) placed it in low-risk category and 1(4.5%) were unaware. Routine preparatory methods such as chemical fixation, centrifugation and preparation of smears were deemed to have higher likelihood of disease by 12(55.6%) people including 6 practicing pathologists, 3 people of academic faculty and 3 postgraduate trainees. While 6(27.8%) comprising of 3 individuals of academic faculty, 2 practicing pathologists and 1 trainee did not agree and 4(16.6%) that is 2 practicing pathologists and 2 academic faculty members were unsure (Table 1).
Table 1: Attitudes of oral pathologists towards donning of appropriate Personal Protective Equipments (PPEs), discarding of samples and variation in laboratory protocols during Covid-19 pandemic.
| Extent of Personal Protective Equipment (PPE) | Histopathology
n=15 |
Cytopathology
n=15 |
Hematology
n=7 |
p-Value |
| Complete PPE | 8 (50%) | 8 (52.4%) | 4(59.1%) | 0.50 |
| Selective PPE with addition or removal of one or more components | 5 (35%)
|
5 (33.3%)
|
2 (31.8%)
|
0.50 |
| N-95 Masks, Head caps and gloves only | 2 (15%) | 2 (14.3%) | 1 (9.1%) | 0.50 |
| Improper discarding of different specimens
Increases risk Of COVID 19 in community |
Yes | No | Unaware | |
| Blood/ plasma | 12(54.54%) | 8(36.36%) | 2(9.09%) | 0.72 |
| Aspirate | 17 (77.27%) | 3(13.64%) | 2(9.09%) | 0.72 |
| Histopathology Chemicals | 10 (45.45%) | 8(36.37%) | 4(18.18%) | 0.72 |
| Variation in laboratory protocol if history suggests COVID 19 positive | Protocol will change (such as extent of PPE) | Protocol will not change (All specimens infectious) | Unsure | |
| 9(40.90%) | 12(54.5%) | 1(4.6%) | 0.51 |
A total of 16 (72.7%) 7 consultants, 5 teachers and 4 residents agreed that they have a bio-safety cabinet (BSC II). That is recommended by CDC for preparation and chemical or heat fixing of smears in their laboratory 2(9.1%) both academic faculty members did not have one and 4(18.2%) which includes 3 practicing pathologist and 1 faculty individual were unaware (Table 1). Since spillage is a probable accident that can occur in the laboratory during routine procedures, questions were asked to evaluate practices in case of one. Thirteen showed a positive view towards immediate reporting; nine people thought that the person being exposed to the spill should be immediately tested for Covid-19. Four respondents suggested isolation for 14 days and two were in support of all options.
Table 2: Knowledge and attitude of pathologists regarding oropharyngeal specimens.
| Knowledge | Practicing Pathologist
n=10 |
Academic Faculty
n=8 |
Postgraduate Trainees n=4 |
| Awareness of modified guidelines | |||
| Yes | 7 | 4 | 4 |
| No | 3 | 4 | 0 |
| Unaware | 0 | 0 | 0 |
| Risk of transmission from oral specimens | |||
| High risk | 8 | 6 | 4 |
| Moderate risk | 0 | 2 | 0 |
| Low risk | 1 | 0 | 0 |
| Unaware | 1 | 0 | 0 |
| Practices | Practicing Pathologist
n=10 |
Academic Faculty
n=8 |
Postgraduate Trainees n=4 |
| Presence of bio-safety cabinet | |||
| Yes | 7 | 5 | 4 |
| No | 0 | 2 | 0 |
| Do not know | 3 | 1 | 0 |
| Well Equipped laboratory | |||
| Present | 6 | 6 | 3 |
| Not present | 2 | 2 | 1 |
| Unaware | 2 | 0 | 0 |
| Laboratory ensures safety of personnel | |||
| Present | 4 | 7 | 4 |
| Not present | 4 | 1 | 0 |
| Unaware | 2 | 0 | 0 |
| Diagnostic benefits from cytopathology procedures outweigh risk of Covid-19 | |||
| Present | 10 | 5 | 1 |
| Not present | 0 | 3 | 2 |
| Unaware | 0 | 0 | 1 |
| Suspension of oral aspirates unless extremely warranted during Covid-19 | |||
| Present | 6 | 4 | 2 |
| Not present | 3 | 3 | 0 |
| Unaware | 1 | 1 | 2 |
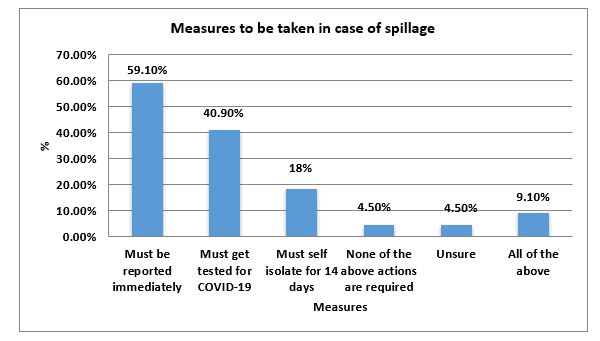
Figure 2: Measures to be taken in case of spillage in the laboratory.
A large number of participants that is 15(68.2%) were sure that their laboratory is well equipped for safe handling practices and spillage contamination (Figure 2), and 3(13.6%) were unaware and p=0.50 indicated an insignificant association between pathologists of the histopathology and hematology lab and their satisfaction regarding laboratory equipment. In addition, 15(68.2%) were hopeful that their laboratory will be able to maintain all the additionally required equipment and chemicals to ensure the safety of personnel, on the contrary 5(22.7%) did not agree and 2(9.1%) pathologists were not aware.
In response to the question if the diagnostic benefits of cytopathology procedures outweigh the risk of virus transmission many of the participants 16(72.7%) were in favor of this fact. 4(18.2%) did not agree and 2(9.1%) were not aware. The pathologists were asked if they were of the opinion that collected aspiration should be withheld during the pandemic unless extremely necessary and 12 agreed, five did not agree and five were unaware (Table 2).
Our study surveyed the knowledge, attitude and practices of pathologists and faculty members in contact with oropharyngeal specimens. Most of the individuals had adequate knowledge regarding the modified guidelines provided by organizations such as WHO and CDC. Adequate knowledge was found regarding the possible modifications in the laboratory setting and difference in transmission of virus due to various procedures. More than 50% participants had a satisfactory attitude regarding the donning of PPEs. Practices were found to be adequate and in line with the provided guidelines.
A questionnaire based study conducted in India by Chettiankandy et al. included individuals working in the histopathology, cytopathology and hematology laboratories as their main respondents reports that 97.4% of the people were in support of changes in layout of laboratory, disinfection of surfaces, maintaining adequate social distancing and hygiene protocol14.
The findings of this study are in line with the perceptions of pathologists, faculty members and PG trainees. The finding also agrees with the specific guidelines provided by CDC for pathology laboratories to combat this health hazard while providing diagnostic facilities, it emphasis on appropriate laboratory space and handling of specimens. The organization recommends proper social distancing along with use of PPEs. A minimum number of individuals should be allowed and virtual contact should be used for external vendors and other personals. Reconfiguration of the laboratory is suggested with reduction in shared equipment. Studies have indicated that the virus can also be carried through data forms used in histopathology/cytopathology laboratories15. All the contaminated surfaces should be thoroughly disinfected after completion of procedures16.
Baskota et al. in their study have found formalin to be useful in inactivating the causative agent of the disease. The study also suggests the use of other fixative materials composed of weaker alcohols17. This finding can be considered adequate since CDC recommends that these samples should be treated as bio-safety level II that the standard level is indicating a mild to moderate health risk when dealing with blood, plasma or another specimen with unknown infectious agent. Most cytopathology specimens are fixed with formalin or 70% alcohol effectively killing the virus with reduce risk of transmission18.
A study in India reported 71.1% of the individuals were of the opinion to suspend aspiration and collection of oral specimens temporarily since potential route of transmission of Covid-19 can be through oral biopsies and smears or samples obtained from periodontal pockets. An increased viral load can be found in the upper respiratory tract necessitating the use of additional precautions in additional to universal that are followed commonly8. In the light of these findings, the knowledge of laboratory personals can be considered adequate. A study conducted in Italy by Vigliar et al. suggested about the decreasing the number of personnel’s in the laboratory along with donning of proper PPE 19.
Preparatory steps that may generate aerosols or droplets include expelling aspirates from the needle or syringe; smearing the aspirated material; and potentially, air-drying or heat drying the smears, in which pathologists, trainees, or cytotechnologists may be involved during rapid on-site evaluation (ROSE). That may result in possible exposure to the virus; hence, these procedures should be done with complete PPE 20,21. Our results highlight sufficient knowledge of these principles among pathologists.
An Asia Pacific survey with an inclusion of 167 laboratories from 24 countries performed to observe the impact of COVID 19 pandemic on histopathology/cytopathology laboratories reports the use of additional PPEs during handling of samples. Thus, 29(17.4%) laboratories provided no additional PPE and 65.6% provided PPEs when handling of all specimens while remaining gave PPEs only when individuals were contact in with samples from the respiratory tract22.
The findings of the study can be considered inadequate since they are contradictory to CDC guidelines, which suggest PPE should include disposable gloves and laboratory coats. Procedures conducted in biosafety cabinet (BSC) should be performed wearing gloves, gown, eye and respiratory protection23. Acceptable methods of respiratory protection include a properly fit-tested, NIOSH-approved filter respirator (N-95 or higher level) or a powered air-purifying respirator (PAPR) equipped with high-efficiency particulate air (HEPA) filters. In addition to use of proper PPEs, there is also need of frequent decontamination of work related stationary, computers, desk and microscopes since the virus can remain viable on surfaces until 72 hours24.
Wang et al. in their study on 167 laboratories reported that 88(52.7%) laboratories had an unchanged laboratory during the pandemic while 79(47.3%) had new equipment installed or modified the previous ones. The results of our study are encouraging in this regard, as most of the individuals have expressed confidence in their laboratory personals being able to deal with circumstances in wake of Covid-19 pandemic. As a institute receives hundreds of samples in the histopathology/cytopathology and hematology laboratories on a daily basis with risk of transmission of virus during collection or transport of sample, therefore the design of the laboratory should be according to WHO guidelines25. The laboratory should be designed for biosafety level (BSC II). The design allows unidirectional flow of air with automated doors and a washbasin. Our results indicate the presence of a biosafety cabinet (BSC II) by most participants.
During several laboratory procedures, spillage can be a common occurrence confirming the fact as stated by the WHO that professional should deal all specimens, spillage of material is possible during sample reception. Adequate risk assessment and risk management under the WHO recommendations follow such events26. A well-trained staff with due knowledge and recognition of standard laboratory measures and also enlightened with modified measures to be taken in light of Covid-19 pandemic are part and parcel of a laboratory providing accurate diagnosis without putting the individuals working over there at risk of death or ill health. The study focuses on the knowledge, attitude and practices of pathologists dealing with oropharyngeal samples in a single institute and finds them to be adequate; however, it is recommended that a wider survey should be conducted to measure the level of knowledge of pathologists and laboratory personals.
The risk of transmission of novel coronavirus is high for pathologists dealing with oropharyngeal specimens and can be a sign of grave danger. The study focuses on a highly specialized group of individuals; therefore, it is a positive factor that knowledge regarding handling and processing of these samples is adequate.
We would like to acknowledge our institution Dow University of Health Sciences.
The authors declare no conflict of interest.
Ethical approval/permission was obtained from the department.
All participants of the study at the start of questionnaire filled an online consent form.
MS conceived the research question and collected the data. MZ supervised the whole project. SZ helped in data collection and manuscript write-up. SM, AU and LA contributed in manuscript writing.
- Ahmed MA, Jouhar R, Ahmed N, Adnan S, Aftab M, Zafar MS, et al. Fear and practice modifications among dentists to combat Novel Coronavirus Disease (COVID-19) outbreak. Int J Environ Res Public Health. 2020;17(8):2821.
- Han Q, Lin Q, Ni Z, You L. Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respir Viruses. 2020; 14(4):470-471.
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577-582.
- Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection. medRxiv [Internet]. 2020 [cited 2020 July 8]. Available from: https://www.medrxiv.org/content/10.1101/2020.02.18.20024539v2
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020; 34:1-14.
- Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TM, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiol. 2020;296(2):E46-E54.
- Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020; 35(20):1-6.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177-1179.
- Güçlü E, Koroglu M, Yürümez Y, Toptan H, Kose E, Güneysu F, et al. Comparison of saliva and oro-nasopharyngeal swab sample in the molecular diagnosis of COVID-19. Rev Assoc Med Bras. 2020;66(8):1116-1121.
- Rossi ED, Pantanowitz L. International perspectives: Impact of the COVID‐19 pandemic on cytology. Cancer Cytopathol. 2020;128(5):1-2.
- Centers for Disease Control and Prevention [Internet]. Interim guidance for healthcare facilities: preparing for community transmission of COVID-19 in the United States 2020 [cited 2020 23 March]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/guidance-hcf.html
- Ahmed N, Hassan W, Rasool R, Fahim U, Shakil A, Khan KS. Knowledge, attitude and practices regarding covid-19 among a cross-sectional sample from Karachi, Pakistan: Descriptive data. J Infect Dis Epidemiol. 2020;6(5):1-12.
- Afzal MS, Khan A, Qureshi UU, Saleem S, Saqib MA, Shabbir RM, et al. Community-based assessment of knowledge, attitude, practices and risk factors regarding COVID-19 among Pakistanis residents during a recent outbreak: a cross-sectional survey. J Commun Health. 2021;46(3):476-486.
- Chettiankandy TJ, Sachdev SS, Sardar MA, Sonawane SG. COVID-19 pandemic: Implications for oral pathologists working in histopathology, cytology and hematology laboratories. Arch Cytolo Histopathol Res. 2020;5(3):219-223.
- Hasan A, Nafie K, Abbadi O. Histopathology laboratory paperwork as a potential risk of COVID-19 transmission among laboratory personnel. Infect Prev Pract. 2020;2(4):1-6.
- Center for Disease Control and Prevention [Internet]. Guidance for general laboratory safety practices during the covid-19 pandemic [cited 24 August 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-safety-practices.html
- Baskota SU, Chandra A, Cross P. The practice of cytopathology during the era of COVID-19: Challenges and changes. Diagn Histopathol. 2020:1-7.
- Pambuccian SE. The COVID-19 pandemic: implications for the cytology laboratory. J Am Soc Cytopathol. 2020;9(3):202-211.
- Vigliar E, Iaccarino A, Bruzzese D, Malapelle U, Bellevicine C, Troncone G. Cytology in the time of coronavirus disease (covid-19): an Italian perspective. J Clin Pathol. 2021;74(4):261-263.
- Centers for Disease Control and Prevention [Internet]. Appendix F5— Laboratory biosafety guidelines for handling and processing specimens associated with SARS-CoV 2020 [cited 23 March 2020]. Available from: https://www.cdc.gov/sars/guidance/f-lab/app5.html
- Chen CC, Chi CY. Biosafety in the preparation and processing of cytology specimens with potential coronavirus (COVID‐19) infection: Perspectives from Taiwan. Cancer Cytopathol. 2020;128(5):309-316.
- Wang YH, Bychkov A, Chakrabarti I, Jain D, Liu Z, He S, et al. Impact of the COVID‐19 pandemic on cytology practice: An international survey in the Asia‐Pacific region. Cancer Cytopathol. 2020;128(12):895-904.
- Centers for Disease Control and Prevention [Internet]. Biosafety in microbiological and biomedical laboratories [cited 23 March 2020]. Available from: https://www.cdc.gov/labs/pdf/CDC-BiosafetyMicrobiologicalBiomedicalLaboratories-2009-P.PDF
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567.
- World Health Organization. Laboratory biosafety manual [Internet]. Available from: https://www.who.int/csr/resources/publications/biosafety/Biosafety7.pdf
- World Health Organization. Laboratory biosafety guidance related to coronavirus disease (COVID-19), 2004 [cited 13 May 2020]. Available from: https://www.who.int/publications/i/item/laboratory-biosafety-guidance-related-to-coronavirus-disease-(covid-19)
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
