By Kanwal Iqbal1, Mervyn Hosein2, Saima Butt1
AFFLIATIONS:
- Department of Oral Pathology, Ziauddin University, Karachi, Pakistan.
- College of Dentistry, Ziauddin University, Karachi, Pakistan.
Oral submucous fibrosis (OSMF) is a premalignant condition, which involves the oral cavity as well as pharynx and upper third of the esophagus. It is a predominant disease of South and South-East Asian population. OSMF has the highest rate of malignant transformation among the other oral premalignant disorders (OPMD) that is 7-30% with a prevalence of about 0.03% to 6.42%. OSMF is described by extensive fibrosis of submucosa and reduced vascularity, which results in compromised blood supply guiding to hypoxia of tissue. This tissue hypoxia brings about the transcription of a set of genes associated with angiogenesis, breakdown of iron/glucose, cell division and cell stability. Hypoxia Inducible Factor-1α (HIF-1α) is the main biomarker intervening in this reaction. The alteration of cells to hypoxia emerges to be mediated through hypoxia inducible factor, which is said to be linked with malignant transformation of epithelium. Research-based studies covered on the probable mechanism of HIF-1α were explored using PubMed and Google Scholar. In this review, we analyzed over 40 articles from the years 2004-2021, which were published regarding the structure and function of HIF-1α, and its association with OSMF. Research articles in which HIF-1α is used separately or in combination with other biomarkers in oral submucous fibrosis have been selected.
Keywords: Biomarker; Oral Submucous Fibrosis; Esophagus; Hypoxia.
Oral Submucous Fibrosis (OSMF) is considered as an insidious, chronic disease that affects any part of the oral cavity and sometimes the pharynx and oesophagus. It is always associated with a juxta epithelial inflammatory reaction followed by fibroelastic change of the lamina propria and epithelial atrophy leading to mucosal stiffness and functional morbidity1-3. The histopathological characteristics include chronic inflammation, excessive collagen deposition in the connective tissues below the oral mucous epithelium, local inflammation in the lamina propria and degenerative changes in the muscles4. Further features of OSMF comprises of dry mouth, pain and taste disorders1,5.
As per the World Health Organization (WHO) figures, there are > 5 million patients of OSMF worldwide6,7. In recent years, the predominance of OSMF has risen from 0.03% to 6.42% with a malignant conversion rate of 7%-30% 8,9. Areca nut was identified as the key etiological agent causing OSMF10,11. Pakistan is one of South Asia’s leading countries where large quantities of areca nut are ingested due to easy accessibility, this leads to the high incidence of OSMF12-14.
Hypoxia is considered as an important factor in the progression to malignant transformation. Extreme fibrosis of the connective tissue result in decreased vascularity leading to tissue hypoxia and resulting in upregulation of HIF-1α 15. After adjusting to low oxygen strain, i.e., tissue hypoxia brings about the change in transcription of a set of genes, which are associated with angiogenesis, breakdown of iron and glucose and cell division. HIF-1α is the basic element mediating this reaction16. HIF-1α was first identified in 1995 and since its detection; it has been associated with several types of disease from cancer to infection. In order to respond to these essential conditions, cells with oxygen levels below physiological levels engage in a variety of biological responses. Hypoxic cells respond by upregulating the expression of number of genes that aid in survival in these conditions17. The purpose of this article is to feature the role of hypoxia in the initiation of cancer formation and to recommend potential inferences for research in this arena.
This review explored the association of a biomarker hypoxia inducible factor-1α in the malignant transformation of oral submucous fibrosis (OSMF).
Structure and Role of Hypoxia Inducible Factor-1α
The hypoxia-inducible factor (HIF-1) is an oxygen-dependent transcriptional accelerator that takes a vital part in angiogenesis of tumor. HIF-1 comprises of HIF-1α and HIF-1β subunit and HIF-1α has also two other subunits (HIF-2α or HIF-3α) 18. The strength and action of HIF-1α is controlled by numerous post-translational modifications, hydroxylation, acetylation and phosphorylation. Thus, HIF-1α links with multiple factors of protein which includes prolyl hydroxylase (PHD), von Hippel-Lindau tumor suppressor gene (pVHL) and E1A-associated protein/ CREB-binding protein (p300/CBP) 19. In normoxic conditions, the HIF-1α is promptly degenerated through pVHL- mediated ubiquitin -proteasome pathway. The association between pVHL and HIF-1α in normoxia is caused by hydroxylation of proline and acetylation of lysine within the oxygen- dependent degradation (ODD) domain segment of polypeptides.
On the other hand, the subunit HIF-1αbecomes immovable in the hypoxic state and associates with protein coactivators, for example, p300/CBP to regulate its transcriptional function20,21. Figure 1 represents the structure of HIF-1α. Eventually, HIF-1α behaves as a chief controller of various hypoxia-inducible genes in hypoxic environment. The target genes of HIF-1α are particularly linked to angiogenesis, cell expansion/endurance and iron and glucose digestion. In addition, HIF-1α stimulation is being reported to be strictly linked to a different variety of tumors and oncogenic paths. Therefore, the impeding of HIF-1α itself or HIF-1α communicating proteins can obstruct tumor development. Therefore, HIF-1α can be selected as a target for antineoplastic remedies based on these data22,23.
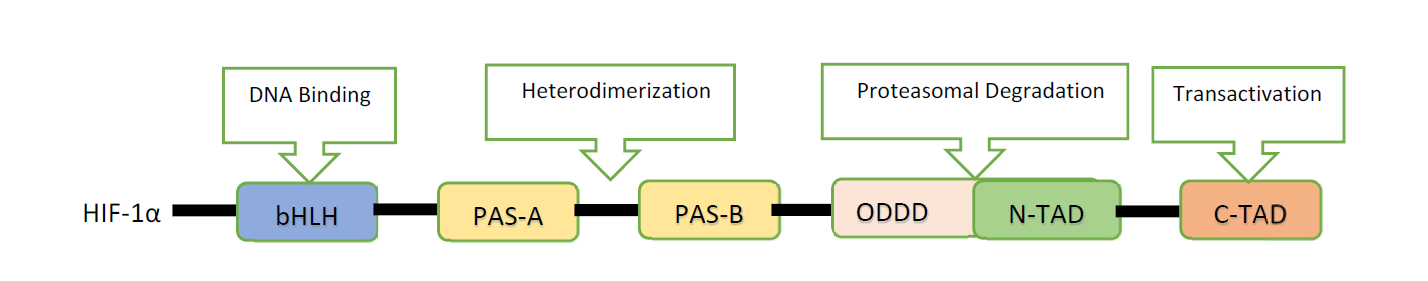
Figure 1: Structure of Hypoxia Inducible Factor-1α (HIF-1α) 23.
Role of Hypoxia in the Progression of Oral Submucous Fibrosis (OSMF)
Hypoxia has been suggested to be a significant micro-environmental factor in oral submucous fibrosis (OSMF) linked to betel quid chewing and its malignant transformation. HIF-1α is the principal regulator of hypoxia in cellular reaction and is highly upregulated in various fibrotic diseases including OSMF24. Articles have likewise shown relationship among hypoxia and fibrosis in lung fibroblasts and renal fibroblasts25,26. Tumor hypoxia is a well-known phenomenon that is induced within the tumor microenvironment by an imbalance between the availability of oxygen and its consumption. Some tumor cells can survive under prolonged hypoxic conditions and adapt themselves to cellular changes caused by hypoxia that can lead to more aggressive phenotype, resulting in invasion and metastasis27. Hyperbaric oxygen treatment (HBO) raises oxygen tension and delivery to oxygen- deficient tissues and may act as a supplementary fibrogenesis therapy involving hypoxia28.
There is a rise in expression of HIF-1α in fibroblasts cells and epithelial cells of renal and lung tissue resultant to hypoxia. The same can be postulated for OSMF connective tissue. Further, overexpression of TGF-β through HIF-1α has also been demonstrated. These observations support the speculation that hypoxia takes part in the advancement of fibrosis in OSMF after the disease activity begins with arecoline present in the betel quid29.
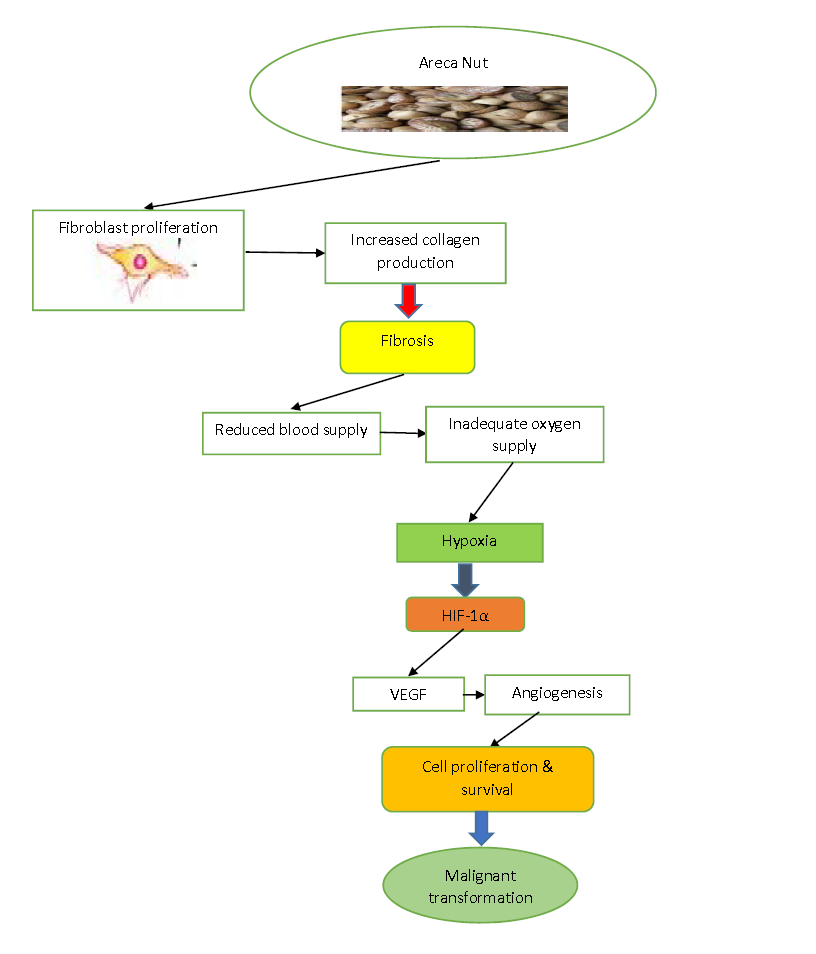
Figure 2: Schematic representation of role of hypoxia in the progression of Oral Submucous Fibrosis (OSMF) initiated by arecanut29.
Cellular Responses to Hypoxia
A variety of responses is exhibited by cells under hypoxic stress, which includes changes in metabolism, expanded angiogenic ability and alterations in cell development and endurance. The master regulator of cell reactions to hypoxia is recognized as HIF-1α. HIF-1α specifically affects the expression of more than 100 target genes, which are associated with various mechanisms such as “angiogenesis, cell metabolism, invasiveness and erythropoiesis.” Vascular endothelial growth factor (VEGF) is additionally over-expressed by hypoxia and HIF-1α is a significant intermediary of angiogenesis30.
Expression of HIF-1α in OSMF
HIF-1α has been reported to be involved in the malignant transformation of OSMF. Extreme fibrosis of the connective tissue results in decreased vascularity leading to tissue hypoxia and resulting in upregulation of HIF-1α. Understanding the mechanism of malignant transformation in the surrounding of OSMF may contribute to prompt identification of oral squamous cell carcinoma (OSCC) 31. HIF-1α is related with the initiation of cancer formation and development of tumor by the modulation of genes, which are associated with various biological mechanisms such as angiogenesis and glycolytic metabolism. HIF-1α, a HIF protein is over-expressed in OSMF which enhances the expression of a variety of other factors, for instance, vascular endothelial growth factor (VEGF) 32. However, very little work has been done to find an association between hypoxia inducible factor-1α and Oral Submucous Fibrosis with different methods of analysis. Table 1 lists all the studies that evaluated expression of HIF-1α in OSMF.
Table 1: Studies that evaluated HIF-1α expression in Oral submucous fibrosis (OSMF).
| Author and Year | Sample Type | Detection Method | Findings |
| Pereira et al. 2020 | 20 biopsy proven cases of Oral submucous fibrosis (OSMF) | Immunohistochemistry (IHC) | Increased expression of HIF-1α in OSMF |
| Gupta et al. 2018 | 150 samples (50 OSMF, 50 Oral squamous cell carcinomas (OSCC), 50 Controls) | Polymerase chain reaction (PCR) | HIF-1α expression in OSMF and OSCC were found to be significantly higher |
| Hande et al. 2019 | 75 histopathologically proven cases of OSMF | Immunohistochemistry (IHC) | HIF-1α increased significantly from no dysplasia to low to high-risk epithelial dysplasia |
| Ho et al. 2017 | 15 OSMF Buccal mucosal fibroblasts (BMFs) and 10 normal BMFs | Cell culture | HIF-1α mRNA expression was significantly higher in OSMF BMFs than normal BMFs
|
| Dalmia et al. 2016 | 15 samples each of Normal oral mucosa (NOM), OSMF and OSCC | Reverse transcription polymerase chain reaction (RT-PCR) | An upregulation of HIF-1α expression is positively correlated with oral carcinogenesis and grade of OSCC |
| Chaudhary et al. 2015 | 20 cases each of OSMF, OSCC and OSCC with OSMF diagnosed histopathologically | Immunohistochemistry (IHC) | Increase in expression of HIF-1αfrom OSMF to OSCC to OSCC with OSMF is noticed |
| Tilakaratne et al. 2008 | 48 samples of OSMF
|
Immunohistochemistry (IHC), Reverse transcription polymerase chain reaction (RT-PCR) | HIF-1αis over-expressed in OSMF at both protein and mRNA levels and the correlation of epithelial dysplasia is statistically important |
Correlation of HIF-1α with Oral Submucous Fibrosis (OSMF)
Pereira et al. have evaluated the qualitative expression of HIF-1α in conversion of oral submucous fibrosis into malignancy. The goal of this research was to assess the expression of HIF-1α in OSMF by immunohistochemistry. A retrospective analysis was conducted which comprised of 20 histopathologically diagnosed cases of OSMF and five subjects were chosen from age and sex coordinated individuals for the control group. Their findings showed increased HIF-1α expression in OSMF and they concluded that HIF-1α seems to have a part in OSMF malignant change33. Tilakaratne et al. have described significant upregulation of HIF-1α in the formalin fixed and frozen samples of OSMF as compared to normal mucosa by using immunohistochemistry and RT-PCR. They have taken 48 blocks of OSMF cases and 10 biopsies of normal oral mucosa. They found out that HIF-1α levels of both protein and mRNA were upregulated in OSMF and the relationship with dysplasia of epithelium was statistically significant (p<0.001) 34.
Researchers also concluded that HIF-1α may contribute to the progression of fibrosis34,35. Another research led by Tsai et al., have differentiated the expression of HIF-1α in tissues of normal buccal mucosa and OSMF cases using immunohistochemistry. HIF-1α expression of fibroblasts that was cultured from tissues of OSMF and normal buccal mucosa was assessed using Western blot. Normal fibroblasts of buccal mucosa were tested by arecoline, which is a significant alkaloid of areca nut to clarify if HIF-1α expression could be influenced by arecoline.
The findings indicated that HIF-1α expression was notably over-expressed in OSMF cases and demonstrated chiefly by cells of fibroblasts, epithelium and inflammation. The results showed rise in expression of HIF-1α protein in fibroblasts cells which was derived from OSMF than normal fibroblasts of buccal mucosa (p<0.005). The aforementioned findings suggested that HIF-1α expression was notably higher in tissues of OSMF in consumers of betel nut, suggesting a possible role as a biomarker of hypoxia in local tissues36. Similarly, Ho et al. differentiated HIF-1α expression from normal fibroblasts cells of buccal mucosa and OSMF specimens and explored probable processes, which instigate HIF-1α expression. They also evaluated the reaction of alkaloid present in arecanut, which is known as arecoline on HIF-1α expression in normal fibroblasts of buccal mucosa in vitro. Buccal mucosal fibroblasts (BMFs) of OSMF showed altogether higher mRNA expression of HIF-1α in comparison with normal fibroblasts of buccal mucosa (p<0.005) and arecoline has been found to upregulate HIF-1α mRNA gene expression in a subordinate way. In ending, they stated that hypoxia through the expression of HIF-1α can provide to the proliferation of fibrous tissue in the progression of OSMF associated with the consumers of betel nut37.
Another study conducted by Joseph et al. investigated HIF-1α expression in fifty-seven samples of OSCC, forty-one samples of Oral epithelial dysplasia (OED) which includes twelve, seventeen and twelve mild, moderate and severe OED samples respectively and fourteen samples of normal oral mucosa (NOM) by using immunohistochemistry. The mean nuclear HIF-1α labeling indices (LIs) were found to increase significantly from NOM to OED (mild to moderate to severe) to OSCC (p<0.001). They found out that there was a strong association among the higher mean nuclear HIF-1α LI and OSCC which was accompanied by large tumor area, regional lymph node metastasis or further developed clinical phases (p<0.001). They concluded that the expression of HIF-1α is an initial incident in formation of oral cancer32. Further in specimens of OSCC, the nuclear HIF-1α LI could predict the progression and survival of patients of OSCC38.
In another study, which was supervised by Gupta et al. observed the frequency of single nucleotide polymorphisms of VEGF and HIF-1α was examined in 150 specimens. That were divided into 3 groups (fifty samples of OSMF, fifty samples of oral cancer and fifty samples of healthy controls) utilizing the polymerase chain reaction (PCR) procedure of the restriction fragment length polymorphism (RFLP). A significant increase in the frequencies of single nucleotide polymorphisms (SNPs) of VEGF and HIF-1αin OSMF and OSCC was found as compared to controls (p<0.001). Therefore, SNPs can also serve as a prognostic biomarker and likewise it can be utilized in the invention of drugs against VEGF or HIF-1α in the treatment as well as in the prevention of OSCC in OSMF39.
Chaudhary et al. investigated the link among the expression of HIF-1α and mean blood vessel density in OSMF, OSCC and OSCC with OSMF by immunohistochemistry. They have taken 20 histopathologically proven samples of OSCC, OSMF alone and OSMF with oral cancer. The immunohistochemistry was carried out on tissue sections, which was embedded in paraffin by utilizing monoclonal antibody of HIF-1α. The increase in the expression of HIF- 1α from OSMF to OSCC to OSCC with OSMF was noticed and blood vessel density was found to be highest in OSCC with OSMF, intermediate for OSCC and lowest for OSMF40.
Another research done by Dalmia et al. stated the hypothesis of differential expression of HIF-1α in invasive conversion of OSMF was examined. Fifteen specimens each of normal mucosa, OSMF and OSCC were taken and their sections were calculated using Hematoxylin and Eosin (H&E) staining, which was fixed in formalin and connection among the differential expression of HIF-1α mRNA, OED and grades of oral cancer, were tested via RT-PCR. It has been figured out that a rise in HIF-1α expression is related positively with carcinogenesis and grades of oral cancer, whereas downregulation is related with fibrosis. Consequently, they concluded that it can be used as both diagnostic and prognostic marker41. Hande et al. evaluated the expression of HIF-1α in OSMF. They included 75 cases of OSMF, which are proven histopathologically, and then analysis was done on different grades of OSMF using immunohistochemistry. After examining in various grades of OSMF for expression of HIF-1α, it was found that there was a remarkable increase in expression from normal oral mucosa to without epithelial dysplasia and from low towards high-risk dysplasia of epithelium. The altered HIF-1α expression can signify that there is a disturbance in epithelial-mesenchymal interaction, which is an indication of OSMF progression towards malignancy. Thus, HIF-1α expression showed good association with increase in epithelial dysplasia grades. Therefore, it can assist in quantifying epithelial dysplasia in OSMF42.
Future Research
There is a fundamental revolution in the research techniques, the production of biologic protein from saliva as compared to tissues or blood. However, there is a need to carry out more studies on saliva samples as it can be utilized as an appropriate tool for identification of HIF-1α levels in patients because of its painless and non-invasive character and has the ability to be emerged as an economical marker of screening in the coming years.
The timely identification of altered HIF-1α expression may provide an opportunity for early identification of possible transformation of oral submucous fibrosis (OSMF) into oral cancer and could help minimize morbidity and mortality due to its potential for malignancy. Likewise, it can be guided adequately as a medium of remedy to arrest the transformation of OSMF into malignancy. In this review, some promising mechanism of action of HIF-1α has been recognized by analyzing previous researches.
All authors are genuinely acknowledged and regarded special thanks to the fellow postgraduate trainees at Ziauddin University for their valuable suggestions towards reviewing this article.
There was no conflict of interest among the authors.
KI did the conceptualization of study, literature search and prepared the write up. MH and SB did the proof reading and evaluated the manuscript thoroughly.
- Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW. Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol Head Neck Surg. 2020;49(1):1-11.
- Tilakaratne WM, Ekanayaka RP, Warnakulasuriya S. Oral submucous fibrosis: a historical perspective and a review on etiology and pathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):178-191.
- Tilakaratne WM, Ekanayaka RP, Herath M, Jayasinghe RD, Sitheeque M, Amarasinghe H. Intralesional corticosteroids as a treatment for restricted mouth opening in oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):224-231.
- Tamgadge S, Tamgadge A. Histopathology of oral submucous fibrosis in third dimension with an additional note on hypothesis of epithelial atrophy. J Microsc Ultrastruct. 2020;8(1):31-34.
- More CB, Rao NR. Proposed clinical definition for oral submucous fibrosis. J Oral Biol Craniofac Res. 2019;9(4):311-314.
- Shih YH, Wang TH, Shieh TM, Tseng YH. Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci. 2019;20(12):1-22.
- Gottipamula S, Sundarrajan S, Moorthy A, Padmanabhan S, Sridhar K. Buccal mucosal epithelial cells downregulate CTGF expression in buccal submucosal fibrosis fibroblasts. J Maxillofac Oral Surg. 2018;17(2):254-259.
- Das M, Manjunath C, Srivastava A, Malavika J, Ameena M. Epidemiology of oral submucousfibrosis: A review. Int J Oral Health Med Res. 2017;3(6):126-129.
- Peng Q, Li H, Chen J, Wang Y, Tang Z. Oral submucous fibrosis in Asian countries. J Oral Pathol Med. 2020;49(4):294-304.
- Kondaiah P, Pant I, Khan I. Molecular pathways regulated by areca nut in the etiopathogenesis of oral submucous fibrosis. Periodontol. 2000. 2019;80(1):213-224.
- Bazarsad S, Zhang X, Kim KY, Illeperuma R, Jayasinghe RD, Tilakaratne WM, et al. Identification of a combined biomarker for malignant transformation in oral submucous fibrosis. J Oral Pathol Med. 2017;46(6):431-438.
- Raffat MA, Hadi NI, Hosein M, Zubairi AM, Ikram S, Akram Z. Differential expression of salivary S100A7 in oral submucous fibrosis. Saudi Dent J. 2019;31(1):39-44.
- Chattopadhyay A, Ray JG. Molecular pathology of malignant transformation of oral submucous fibrosis. J Environ Pathol Toxicol Oncol. 2016;35(3):193-205.
- Hegde S, Anuradha A, Asha V. Malignant transformation of oral submucous fibrosis. J Med Radiol Pathol Surg. 2015;1(5):32-36.
- Chatterjee R, Ghosh B, Mandal M, Nawn D, Banerjee S, Pal M, et al. Pathophysiological relationship between hypoxia associated oxidative stress, Epithelial-mesenchymal transition, stemness acquisition and alteration of Shh/Gli-1 axis during oral sub-mucous fibrosis and oral squamous cell carcinoma. Eur J Cell Biol. 2021;100(1):1-11.
- Liu Y, Zhu X, Zhou X, Cheng J, Fu X, Xu J, et al. Different polymorphisms in HIF-1α may exhibit different effects on cancer risk in Asians: evidence from nearly forty thousand participants. Aging (Albany NY). 2020;12(21): 21329-21343.
- Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):553-562.
- Tang W, Zhao G. Small molecules targeting HIF-1α pathway for cancer therapy in recent years. Bioorg Med Chem. 2020;28(2):1-82.
- Santos SA, Andrade Júnior DR. HIF-1alpha and infectious diseases: a new frontier for the development of new therapies. J Inst Trop Med São Paulo. 2017;1-10.
- Jin X, Dai L, Ma Y, Wang J, Liu Z. Implications of HIF-1α in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 2020;20(1):1-11.
- Albanese A, Daly LA, Mennerich D, Kietzmann T, Sée V. The role of hypoxia-inducible factor post-translational modifications in regulating its localisation, stability, and activity. Int J Mol Sci. 2021;22(1):1-17.
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1) α: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1-12.
- Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378-389.
- Phulari RG, Dave EJ. A systematic review on the mechanisms of malignant transformation of oral submucous fibrosis. Eur J Cancer Prev. 2020;29(5):470-473.
- Senavirathna LK, Huang C, Yang X, Munteanu MC, Sathiaseelan R, Xu D, et al. Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Sci Rep. 2018;8(1):1-16.
- Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, et al. Signalling pathways involved in hypoxia‐induced renal fibrosis. J Cell Mol Med. 2017;21(7):1248-1259.
- Ehtisham M, Gupta S, Khan S. Expression of glut-1 in oral epithelial dysplasia and OSCC: An immunohistochemical study. Int J Contemp Med Res. 2020;7(9):15-19.
- Ye X, Zhang J, Lu R, Zhou G. HBO: A possible supplementary therapy for oral potentially malignant disorders. Med Hypotheses. 2014;83(2):131-136.
- Hande AH, Chaudhary MS, Gawande MN, Gadbail AR, Zade PR, Bajaj S, et al. Oral submucous fibrosis: An enigmatic morpho-insight. J Cancer Res Ther. 2019;15(3): 463-469.
- Kujan O, Shearston K, Farah CS. The role of hypoxia in oral cancer and potentially malignant disorders: a review. J Oral Pathol Med. 2017;46(4):246-252.
- Ekanayaka RP, Tilakaratne WM. Oral submucous fibrosis: Review on mechanisms of pathogenesis and malignant transformation. J Carcinog Mutagen. 2013:1-11.
- Joseph I, Elizabeth J, Rao UK, Ranganathan K. Study of hypoxia-inducible factor-2α expression in the malignant transformation of oral submucous fibrosis. J Oral Maxillofac Pathol. 2020; 24(1):33-39.
- Pereira T, Surve R, Shetty S, Gotmare S. Qualitative expression of hypoxia-inducible factor-1α in malignant transformation of oral submucous fibrosis: An immunohistochemical study. J Oral Maxillofac Pathol. 2020; 24(1):106-112.
- Tilakaratne W, Iqbal Z, Teh M, Ariyawardana A, Pitiyage G, Cruchley A, et al. Upregulation of HIF‐1α in malignant transformation of oral submucous fibrosis. J Oral Pathol Med. 2008;37(6):372-377.
- Arakeri G, Patil SG, Aljabab AS, Lin KC, Merkx M, Gao S, et al. Oral submucous fibrosis: an update on pathophysiology of malignant transformation. J Oral Pathol Med. 2017;46(6):413-417.
- Tsai CH, Lee SS, Chang YC. Hypoxic regulation of plasminogen activator inhibitor‐1 expression in human buccal mucosa fibroblasts stimulated with arecoline. J Oral Pathol Med. 2015;44(9):669-673.
- Ho YC, Yang SF, Lee SS, Chang YC. Regulation of hypoxia-inducible factor-1α in human buccal mucosal fibroblasts stimulated with arecoline. J Formos Med Assoc. 2017;116(6):484-487.
- Lin PY, Yu CH, Wang JT, Chen HH, Cheng SJ, Kuo MY, et al. Expression of hypoxia‐inducible factor‐1α is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol Med. 2008;37(1):18-25.
- Gupta S, Sharma A, Gupta N, Mani K, Kharbanda OP. Single nucleotide polymorphisms of VEGF and HIF-1 Alpha in oral submucous fibrosis: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126(4):1.
- Chaudhary M, Bajaj S, Bohra S, Swastika N, Hande A. The domino effect: Role of hypoxia in malignant transformation of oral submucous fibrosis. J Oral Maxillofac Pathol. 2015; 19(2):122-127.
- Dalmia A, Hazarey V, Talkal R, Ganvir S, Purohit HJ, Gupta S, et al. Role of Hypoxia Inducible Factor-1α messenger RNA expression in malignant transformation of oral submucous fibrosis: A RT-PCR study. Transl Res Oral Oncol. 2016;1:1-5.
- Hande AH, Chaudhary MS, Gadbail AR, Zade PR, Gawande MN, Patil SK. Role of hypoxia in malignant transformation of oral submucous fibrosis. J Datta Meghe Inst Med Sci Univ. 2018;13(1):38-43.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
