By Inayat Ali Khan1, Abdul Sami Qureshi2, Atiq Ahmed Khan1, Muhammad Adil Khatri1, Muhammad Sameer Khulsai1, Farhad Ali1
AFFLIATIONS:
- Department of Neurosurgery, Dr. Ziauddin Hospital (North Campus), Karachi, Pakistan.
- Department of Emergency Medicine, Dr. Ruth K. M. Pfau, Civil Hospital Karachi, Pakistan.
Background: Hyponatremia is considered as a common electrolyte imbalance in hospitalized patients. Cerebral salt wasting syndrome (CSWS) is an abnormality in the renal sodium transport in patients with intracranial disease, in the face of preserved thyroid and adrenal function. However, treatment of CSW and syndrome of inappropriate antidiuretic hormone (SIADH) varies, making an accurate diagnosis significant. In order to differentiate CSWS from syndrome of inappropriate antidiuretic hormone (SIADH), this study aimed to detect CSWS related hyponatremia (benign and reversible condition) in its early stages.
Methods: This retrospective cross-sectional study was conducted at the Department of Neurosurgery, Ziauddin Medical University Hospital, Karachi, Pakistan from August 2016 to August 2019. All patients who had a cerebral lesion along with hyponatremia and natriuresis were included. Patients with SIADH, though considered in the differential diagnosis, were not included in the study. Data was analyzed by using SPSS (Statistical Package for the Social Sciences) and p<0.05 was considered statistically significant.
Results: A total of 39 patients in a period of three years were found with CSWS-related hyponatremia, predominantly males 28(71.8%), who were operated for cranial tumors 15(38.46%). All 39(100%) had an increased uric acid level. After treatment 31(79.5%) developed post-florinef hypokalemia, while, potassium remained unaffected in 8(20.5%). Hypokalemia was noted in majority of the patients 31(79.5%) treated with fludrocortisones.
Conclusion: Cerebral salt wasting syndrome was found high among males with cranial tumors. Hyponatremia in CSWS patients was found challenging if untreated might lead to mortality. However, treatment with intravenous normal saline and fludrocortisone was successful in this course.
Keywords: Hyponatremia; Natriuresis; Hypovolemia; Syndrome.
The first description of cerebral salt wasting syndrome (CSWS) was brought to light in 1950 when it was described by Peter.1 just seven years later in 1957 Schwartz et al. reported the syndrome of inappropriate antidiuretic hormone (SIADH) 2. CSWS is associated with a decreased ECF volume and serum sodium levels, increased urinary sodium level and urine output, increased atrial natriuretic peptide (ANP) level and increased brain natriuretic peptide (BNP) levels. Some kind of disturbance in the sodium (Na+) and water balance can be expected in neurosurgical patients following a cerebral insult. This disturbance could potentially lead to hyponatremia, natriuresis and volume depletion leading to what is known as cerebral salt wasting syndrome (CSWS) 1.
Hyponatremia in the setting of cerebral insult should not be taken plainly as hyponatremia itself and that always CSWS and SIADH should be included in the differential diagnosis. It becomes vital for us clinicians to differentiate CSWS from the syndrome of inappropriate secretion of antidiuretic hormone (SIADH), a condition in which there is hyponatremia, natriuresis and concentrated urine and was described by Schwartz et al. in 1957 2. The treatment strategies for these two conditions differ markedly, in CSWS patients are treated with fluids and sodium supplements whereas in SIADH fluids are restricted3.
The consequences of undiagnosed or untreated hyponatremia could be serious resulting in headache, altered consciousness that results in a fall in the Glasgow coma score (GCS), worsening brain edema, and increased severity of hyponatremia, seizures and sometimes death4. The fact is well known that Na+ (Sodium) is the most abundant cation of the human extracellular fluid (ECF) and the amount of Na+ in the body is the most important determinant of the ECF volume5.
Multiple regulatory mechanisms have evolved to control the excretion of this ion and through the operation of these mechanisms, the amount of Na+ excreted is adjusted to equal the amount ingested and a person stays in Na+ balance6. In a neurosurgical setting of patients with cerebral lesions, we noticed that a fall in the GCS was usually attributed to a cerebral cause where suspicion of an increase in the size of a hematoma, increased brain edema etc. in patients. For such conditions, there must be a repeat CT brain, which showed no new significant changes compared to the last CT scan.
Hyponatremia, in its capacity, is an electrolyte disorder that is commonly seen in clinical practice associated with the central nervous system (CNS) disease and delay in diagnosis could at times be potentially fatal7. In the light of this threat, the purpose of our study was to highlight the importance of CSWS, its early diagnosis, differentiation from SIADH and delve into its early management
The study was conducted at the Department of Neurosurgery, Ziauddin Medical University Hospital, (North Campus), Karachi-Pakistan. This was a retrospective cross-sectional study and was conducted from 25 August 2016 to 24 August 2019. Diagnosing cerebral salt wasting syndrome (CSWS) accurately is of considerable importance as any misdiagnosis could lead to negative clinical consequences7.
Our purpose was to determine the incidence and frequency of CSWS in patients with cerebral lesions in the light of potentially fatal hyponatremia and to determine the treatment outcome of such patients, while simultaneously emphasizing the vital importance of differentiation between CSWS and syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Hence, all those patients were included in the study who was 18 years and above, that had a cerebral lesion along with hyponatremia and hypovolemia with a large natriuresis.
Our patients with a cerebral lesion who were diagnosed to have CSWS were ones having head trauma, brain hemorrhage, those who underwent craniotomy for conditions other than brain tumors (like extradural hematoma etc.), patients who operated for brain tumors and those with bacterial meningitis. The patients with CSWS had a urine output of more than three liters over 24 hours (polyuria), serum sodium of less than 130mEq/L, high urine specific gravity (≥1020) and a urinary sodium level ≥40mEq/L. Patients below the age of 18 years were excluded; patients with SIADH, polydipsia, renal disease, use of diuretics, heart failure, hypothyroidism, hormone deficiency and pseudohyponatremia were also excluded from the study.
We calculated the serum osmolality in patients by using the simple formula of 2 x Na+ (Sodium), otherwise, the actual formula: 2 x Na+ + glucose/18 + BUN/2.8 (blood urea nitrogen) 8. We analyzed the data by using SPSS (Statistical Package for the Social Sciences) and p<0.05 was taken to be statistically significant. Once a diagnosis of CSWS was made, our treatment strategies were targeted towards handling the primary brain lesion and CSWS simultaneously. Volume repletion was started immediately by giving the patient isotonic saline as it helped to provide fluid for the hypovolemic patients and restored the body’s sodium levels. In moderate to severe cases 3%, hypertonic saline was used to replenish the volume and sodium levels9.
The levels of serum sodium were being monitored to avoid its overcorrection as this could have led to lethargy, muscle twitching and at times seizures and death10. We were aware of central pontine myelinolysis (CPM) had sodium been corrected too fast, hence swift correction of Na+ was as a rule avoided11. Patients having hyponatremia for at least 2 days, or in those patients where the duration was not known, the rate of rising in the serum sodium concentration was kept at or below 10 mmol/L during any 24 hours, if possible11,12. Additionally, fludrocortisone (florinef) was administered making sure the dose did not exceed 1mg/d. Florinef helps to reabsorb sodium and water from the distal renal tubules13.
This study was conducted at Ziauddin hospital (North campus) in the three-year study period. We had around 39 patients of which 15(38.46%) patients were noted to have CSWS with cranial trauma, 10(25.64%) of them after craniotomy other than brain tumors, 4(10.25%) with operated brain tumors, 3(7.69%) with bacterial meningitis and 7(18.0%) of them with intracranial hemorrhage (Table 1).
Table 1: The incidence of various categories of brain lesions in patients with significant hyponatremia.
| Variables | Number of patients with hyponatremia n(%) | Diabetes
n(%) |
Hypertension
n(%) |
Levels of nitrogen breakdown and K+ |
p-Value |
|
| Males |
28(72%) |
8(28.6%) |
10(35.7%) |
U/acid ↑
|
K+
23 (in all) |
< 0.05 |
| Females |
11(28%) |
5(45.4%) |
6(54.54%) |
↑
|
08
(in all) |
< 0.05 |
| Cranial tumors | 15 (38.46%) | 1(6.7%) | 2(13.3%) | ↑ | 12 | < 0.05 |
| Craniotomy, other than brain tumors | 10 (25.64%) | 3(30%) | 3(30%) | ↑ | 08 | < 0.05 |
| Brain hemorrhage | 7(18.0%) | 6(85.71%) | 7(100%) | ↑ | 06 | < 0.05 |
| Operated brain tumors | 4(10.25%) | 3(75%) | 4(100%) | ↑ | 03 | < 0.05 |
| Bacterial Meningitis | 3(7.69%) | nil | nil | ↑ | 02 | < 0.05 |
The gender distribution was such that 28(72%) were males and 11(28%) were females (Table 1). The age distribution showed that there was almost a general decade wise increasing trend. The highest numbers of patients 15(38.46%) were seen in the 7th decade and above. Since, 4(10.25%) patients each were seen in the 3rd and 4th decade, 7(18.0%) patients were in the 5thdecade and 9(23.07%) patients were in the 6th decade (Figure 1). Although our study involved patients of 18 years and above, we still did not see any patients between 18-20 years. The youngest patient in our study noted to have CSWS was 21 years old. The mean age at the time of admission was 53+0.32.
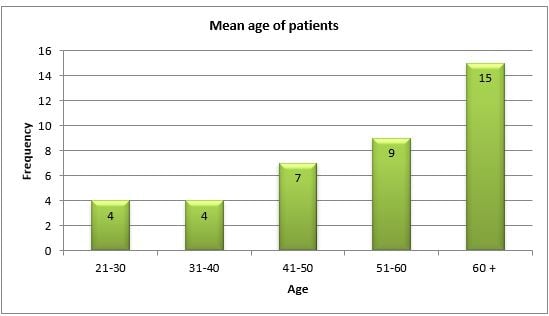
Figure 1: Mean age of the patients in the research study.
A strange correlation was noted between CSWS and raised uric acid levels. All 39 patients had a raised uric acid level (Table 2). This could be contrary to hypouricemia seen in other studies of CSWS14, while 31(79.5%) of them had a fall in the potassium levels (post florinef) (Table 2). Fall in the Glasgow coma score (GCS) with confusion being prominent, polyuria (defined as over three liters of urine output over 24 hours in an adult) with an obvious negative water balance and a low blood pressure was seen in all patients15.
Table 2: Comparison of age with other co-morbidities para to Nitrogen and K+ breakdown Levels.
| Age Groups | Number of patients with hyponatremia n(%) | Diabetes
n(%) |
Hypertension
n(%) |
Other co-morbidities | Levels of nitrogen breakdown
(uric acid) |
Levels
of K+ (hypokalemia) |
| 21-30 | 4(10.25%) | None | None | None | ↑ | 02 |
| 31-40 | 4(10.25%) | None | 1(25%) | None | ↑ | 03 |
| 41-50 | 7(18.0%) | 2(28.6%) | 1(14.3%) | None | ↑ | 05 |
| 51-60 | 9(23%) | 4(44.4%) | 5(55.5%) | Asthma (2)
R/arthritis (1) |
↑ | 08 |
| 60+ | 15(38.46%) | 7(46.7%) | 9(60%) | CABG (1)
TIA (1) |
↑ | 13 |
*CABG= Coronary artery bypass graft; **TIA=Transient ischemic attack, less than 0.05 is significant
The urinary sodium was >40 mEq/L, which again implied a negative nitrogen balance. Other signs of hypovolemia other than hypotension included a lack of skin turgor and an elevated hematocrit. The serum osmolality was low and the urine osmolality was high. Of the 39 patients 13(33.3%) were diabetic and 16(41%) were hypertensive (Table 2). Interesting to note was the fact that our patients developed CSWS between 4-9 days after the ictus. The highest number of patients 28(71.8%) was seen between the sixth to the eighth day.
In this study, we found cerebral salt wasting syndrome (CSWS) and hyponatremia in majority males 28(71.8%) and significantly noted in patients operated for cranial tumors 15(38.46%). As we were fully aware of this threat and its potentially threatening consequences, early diagnosis and aggressive management was the key behind early successes achieved. Cerebral salt wasting syndrome has always been thought of as an under-diagnosed cause of hyponatremia occurring in the setting of intracraniallesions14.
Many studies have described various aspects of CSWS, but the actual pathogenesis of renal salt wasting resulting from a cerebral cause remains to be fully understood15, a strong postulate however contends that an impaired sympathetic neural input could be the reason behind reduced reabsorption of sodium and urate from the proximal tubules16. However, syndrome of inappropriate antidiuretic hormone (SIADH) may be a more common cause of hyponatremia when compared to CSWS17. Urine sodium typically exceeds 40 mEq/L. It may not be easy at all times to pin down the exact incidence and prevalence of CSWS17.
The depressed levels of serum uric acid levels in SIADH are reflective of an increased extracellular fluid (ECF) volume18. By contrast, however a drop in the level of the ECF volume may show a rise in the uric acid levels as was seen in our study. General clinical findings that support a diagnosis of CSWS include dry mucous membranes, orthostatic changes in blood pressure and pulse and flat neck veins19. A review of the patient’s hospital flow sheet would easily indicate a negative fluid balance and this could provide strong evidence to support a fall in the ECF volume19.
While correcting the hyponatremia, constant monitoring of the serum sodium levels becomes imperative as overcorrection may lead to hypernatremia, which can cause muscle twitching, lethargy, seizure and at times death20. Additionally, hyponatremia should not be corrected too quickly. There is always a risk of developing a condition called central pontine myelinolysis if the hyponatremia is corrected too quickly21.
Fludrocortisone (florinef) can be used in doses of 0.1 to 1mg/day to treat the CSWS. It acts by stimulating reabsorption of sodium and water in the distal tubule, which leads to expansion of the intravascular volume22. Florinef acetate is an extract of the natural form of glucocorticoid, which works to maintain the salt and water balance while at the same time keeping the blood pressure in a balanced state. The main pharmacologic target of this drug remains adrenocortical insufficiency23. The drug is available in tablet form for oral use and can be crushed and administered via a nasogastric tube in unconscious patients. The drug is contraindicated if the subject is hypertensive, has an established allergy to the drug, is diagnosed to have high albumin levels in the blood or suffers from any known systemic fungal infection. The patient would require close monitoring with special reference to acute skin eruptions (rash), breathing difficulty, hypertension and facial swelling24.
It may not always be easy to put an exact period as to when fludrocortisone should be commenced22. It has been suggested that this could be considered when the diagnosis has been established and management by replenishing salt and fluids is not readily achieved or is causing practical difficulties22. Common adverse effects associated with fludrocortisone include hypokalemia and hypertension25.
Brain natriuretic peptide (BNP) in humans is found in the brain and the ventricles of the heart. The two postulates of an increased release of BNP from the heart and brain that come from a stress response to an underlying illness and raised intracranial pressure respectively26. Some authors may surmise that the renal salt wasting and the resultant volume depletion in the face of intracranial disease is a protective measure that puts restraints on extreme rises in the intracranial pressure27. Replacement of urine salt and replenishing water losses with 0.9% or 3% sodium chloride is the cornerstone of CSWS management28.
Hyponatremia is a diagnostic challenge for a neurosurgeon in the setting of an acute cerebral lesion, which was seen most commonly among males. CSWS is definitely not a rare condition and it is important to establish an early diagnosis for a better outcome based on the biochemical and clinical profile. Its difference from SIADH is vital as the two conditions are treated differently.
The authors would like to acknowledge the hospital staff and employees of the records department who devoted their time and facilitated us.
The authors declare no conflict of interest regarding the publication of this paper.
The study was approved from the ethical board of Lahore College of Physical Therapy (ERC-LCPT/ 458-3029).
As a routine, consent was obtained from all patients or their first of kin (where patients were not in a position to give their consent)
All authors were equally involved in drafting, literature search, and writing of the paper.
- Peters JP. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians. 1950;63:57-64.
- Schwartz WB, Bennett W, Curelop S, Bartter FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957;23(4):529-542.
- Oh JY, Shin JI. Syndrome of inappropriate antidiuretic hormone secretion and cerebral/renal salt wasting syndrome: similarities and differences. Front Pediatr. 2014;2:1-5.
- Yee AH, Burns JD, Wijdicks EF. Cerebral salt wasting: pathophysiology, diagnosis, and treatment. Neurosurg Clin. 2010;21(2):339-352.
- Pivovarov AS, Calahorro F, Walker RJ. Na+/K+-pump and neurotransmitter membrane receptors. Invert Neurosci. 2019;19(1):1-6.
- Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na, K-ATPase isoforms in health and disease. Front Physiol. 2017;8:1-16.
- Leonard J, Garrett RE, Salottolo K, Slone DS, Mains CW, Carrick MM, et al. Cerebral salt wasting after traumatic brain injury: a review of the literature. Scand J Trauma Resusc Emerg Med. 2015;23(1):1-7.
- Purssell RA, Pudek M, Brubacher J, Abu-Laban RB. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann Emerg Med. 2001;38(6):653-659.
- Fraser JF, Stieg PE. Hyponatremia in the neurosurgical patient: epidemiology, pathophysiology, diagnosis, and management. Neurosurg. 2006;59(2):222-229.
- Hannon MJ, Finucane FM, Sherlock M, Agha A, Thompson CJ. Disorders of water homeostasis in neurosurgical patients. J Clin Endocrinol. 2012;97(5):1423-1433.
- Rao PB, Azim A, Singh N, Baronia AK, Kumar A, Poddar B. Osmotic demyelination syndrome in intensive care unit. Indian J Crit Care Med. 2015; 19(3):166-169.
- Giuliani C, Peri A. Effects of hyponatremia on the brain. J Clin Med. 2014;3(4):1163-77.
- Mori T, Katayama Y, Kawamata T, Hirayama T. Improved efficiency of hypervolemic therapy with inhibition of natriuresis by fludrocortisone in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1999;91(6):947-952.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. Can Med Assoc J. 2002;166(8):1056-1062.
- Betjes MG. Hyponatremia in acute brain disease: the cerebral salt wasting syndrome. Eur J Intern Med. 2002;13(1):9-14.
- Spatenkova V, Bradac O, de Lacy P, Skrabalek P. Polyuria in relation to dysnatraemias in neurocritical care. Br J Neurosurg. 2015;29(5):650-654.
- Sherlock M, O’Sullivan E, Agha A, Behan LA, Rawluk D, Brennan P, et al. The incidence and pathophysiology of hyponatremia after subarachnoid hemorrhage. Clin Endocrinol. 2006;64(3):250-254.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Cerdà-Esteve M, Cuadrado-Godia E, Chillaron JJ, Pont-Sunyer C, Cucurella G, Fernández M, et al. Cerebral salt wasting syndrome. Eur J Intern Med. 2008;19(4):249-254.
- Rahman M, Friedman WA. Hyponatremia in neurosurgical patients: clinical guidelines development. Neurosurg. 2009 ;65(5):925-936.
- George JC, Zafar W, Bucaloiu ID, Chang AR. Risk factors and outcomes of rapid correction of severe hyponatremia. Clin J Am Soc Nephrol. 2018;13(7):984-992.
- Rabinstein AA, Wijdicks EF. Hyponatremia in critically ill neurological patients. Neurologist. 2003;9(6):290-300.
- Polito A, Hamitouche N, Ribot M, Polito A, Laviolle B, Bellissant E, et al. Pharmacokinetics of oral fludrocortisone in septic shock. Br J Clin Pharmacol. 2016;82(6):1509-1516.
- Bamberg K, William-Olsson L, Johansson U, Jansson-Löfmark R, Hartleib-Geschwindner J. The selective mineralocorticoid receptor modulator AZD9977 reveals differences in mineralocorticoid effects of aldosterone and fludrocortisone. J Renin Angiotensin Aldosterone Syst. 2019;20(1): 1-6.
- Momi J, Tang CM, Abcar AC, Kujubu DA, Sim JJ. Hyponatremia—what is cerebral salt wasting? Perm J. 2010;14(2):62-65.
- Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, et al. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid hemorrhage. Lancet. 1997;349(9047):245-249.
- Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-203.
- Guerrero R, Pumar A, Soto A, Pomares MA, Palma S, Mangas MA, et al. Early hyponatraemia after pituitary surgery: cerebral salt-wasting syndrome. Eur J Endocrinol. 2007;156(6):611-616.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
