By Waqas Shabbir1, Kiran Namoos2, Anila Jaleel3, Rukhshan Khurshid4, Sadaf Saleem Uppal5
AFFILIATIONS:
- Department of Gastroenterology, Lahore General Hospital, Lahore, Pakistan.
- Department of Biochemistry, Shalamar Medical and Dental College, Lahore, Pakistan.
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing novel coronavirus (COVID-19) outbreak has an impact on the liver enzymes. The study aimed to determine the serum markers and liver enzymes and their association in COVID-19 patients admitted to hospital.
Methods: It was a retrospective, observational study conducted at the Gastroenterology Department of Lahore General Hospital, from April 2020-July 2020. Total 495 patients were divided into two groups. Group A (318) comprised of patients with moderate symptoms and group B (177) with severe symptoms. Records were obtained from the hospital laboratory in which Lactate dehydrogenase (LDH), Aspartate aminotransferase, (AST), Alanine aminotransferase (ALT), Gamma glutamyl transferase (GGT) and Alkaline phosphatase (ALP) were assessed using an automated biochemistry analyzer. Serum C-reactive protein test was performed by the immunoturbidimetric assays. Serum Ferritin was determined using the ELISA technique.
Results: The mean age of moderate cases was 48.57±16.84 years and of severe cases were 62.51±9.76 years. In both moderate and severe cases, males [200(63%), 98(55.36%)] were more affected than females [118(37.10%), 79(44.63%)]. Furthermore, 79(16%) of severe cases were associated with medical profession. The levels of CRP, Ferritin and LDH were increased significantly in severe COVID-19 cases (p<0.05) compared to moderate ones. On the other hand, liver enzymes ALT, AST, GGT and ALP were non-significantly raised in severe cases compared to moderate cases (p> 0.05).
Conclusion: Serum ferritin, C-reactive protein and lactate dehydrogenase acts as prognostic biochemical parameters based on severity. In addition, levels of liver enzymes were also raised indicating liver derangement; however, the results were not significant.
Keywords: Ferritin; C-Reactive Protein; Lactate Dehydrogenase; Gama Glutamyl Transferase; Covid-19.
An outbreak of mysterious pneumonia and the worldwide spread of this disease were due to novel coronavirus, named “severe acute respiratory syndrome coronavirus (COVID-19)” which become a pandemic1. Most patients infected with COVID-19 presented with fever, dry cough, loss of taste sensation and myalgias. COVID-19 mediated problem suggested the development of inflammatory niche in the structure of alveolar part of the lung that stimulates the process of disease2. A severe respiratory disorder was observed in 10-14% of cases which indicates infiltration and the need for ventilation. Gender, old age and comorbidities such as diabetes and cardiovascular disease are among the major risk factors for the development of severe disease3.
Various biomarkers are currently being examined to find their role in the diagnosis, prognosis and severity of COVID-19. Among these are Ferritin, C-Reactive Protein (CRP), Lactate Dehydrogenase (LDH) and liver enzymes. Serum ferritin is an indicator of body iron status and has a role in inflammation4. Additionally, ferritin has a protective role as it limits the iron supply to the microbes and controls the production of cytokines5. Values of CRP rise in COVID-19 due to the increase in the synthesis of cytokines, which may damage lung tissues. It is proposed that the levels of CRP were directly related to lesions of the lung and may reflect the severity of disease6.
High values of LDH in COVID-19 patients indicate severe/fatal progression of disease. Increased levels of LDH were related to a six-fold upsurge in odds of emerging severe ailment and a sixteen-fold rise in odds of death in cases of COVID-19. It is proposed that severe COVID-19 infections may be a result of cytokine-mediated damage of tissue and release of LDH. In severe form of COVID-19, large amount of isozyme LDH 3 present in tissues of lung release in blood circulation indicating severe type of interstitial pneumonia, usually evolving into respiratory distress syndrome, may act as a marker of the disease7.
At least one half of the patients presented with COVID-19 had extra pulmonary clinical manifestations. Raised values of liver enzyme also showed a link with the severity of disease. Studies showed that 15% to 45% of patients reported abnormal liver enzymes, resulting in liver damage due to infection8. Pathogenesis of COVID-19 suggests the angiotensin converting enzyme 2 receptor as a mark for cell entry. A receptor for angiotensin-converting enzyme (ACE) is present on the endothelial cells of the bile duct and liver, thus making liver a potential organ to get infected by virus9. Many factors are related to the severity of COVID-19 infection and mortality in association with changes of biochemical parameters, during the stay in the hospital, which may affect prognosis of disease. Therefore, the study aimed to determine the levels of various biomarkers and liver enzymes in patients with moderate and severe cases of COVID-19 admitted in a tertiary care hospital.
The study was conducted as a retrospective, observational study, from April 15th to July 31st, 2020 after taking ethical approval (ERC number: 00138-20) at the Gastroenterology Department of Lahore General Hospital. Data of all enrolled patients were obtained from medical records. A total of 495 patients with moderate to severe symptoms of COVID-19 were included in the study. Verbal and written informed consents were obtained from all patients. Patients were divided into two groups based on the severity of the infection. The degree of COVID-19 infection was categorized into moderate and severe based on diagnosis and treatment protocol for Novel Coronavirus Pneumonia (Trial Version 7). Moderate cases were showing fever and respiratory symptoms with radiological findings of pneumonia and severe cases were those having respiratory distress (≥30 breaths/min); oxygen saturation ≤93% at rest and arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa) 10.
Patients with moderate infection of COVID-19 were considered as group A and patients with severe infection of COVID-19 as group B. Patients having known liver disease and any metabolic disease were excluded from the study. Records of biochemical parameters were obtained from LGH laboratory including Lactate dehydrogenase (LDH), Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Gamma glutamyl transferase (GGT) and Alkaline phosphatase (ALP), which were assessed using the HITACHI automated biochemistry analyzer. Serum CRP test was performed by the immunoturbidimetric method. Ferritin was measured using a chemiluminescent microparticle immunoassay on the Alinity platform (Roche Diagnostics).
The data was analyzed by SPSS 23. Quantitative variables were expressed as mean, standard deviation and standard error. Variables were compared by using an independent t-test. Multiple logistic regression was applied to find the association of various parameters with the severity of Covid-19 infection. p-value of < 0.05 was considered statistically significant.
As shown in Table 1, the mean age of moderate COVID-19 cases was 48.57± 16.84 years and of severe cases was 62.51 ± 9.76 years. Among severe cases, 55.36 % were male and 44.63% were female. However, among moderate cases 62.89 % were male and 37.10% were female. In severe cases, 4.52% were associated with medical profession and 95.48 % were related with non-medical profession and in moderate cases 15.73% belonged to the medical profession and 84.27% had non-medical profession.
Table 1: Descriptive variables of patients of group A (moderate infection) group B (severe infection of COVID-19).
| Variables | Group A, n=318
n(%) |
Group B, n=177
n(%) |
|
| Age (years), Mean±SD | 48.57±16.84 | 62.51±9.76 | |
| Males | 200(63%) | 98(55.36%) | |
| Females | 118(37.10%) | 79(44.63%) | |
| Occupation | Medical | 50(15.73%) | 8(4.52%) |
| Non-medical | 268(84.27%) | 169(95.48%) | |
| Residence | urban | 227(71.38%) | 105(59.32%) |
| rural | 91(28.61%) | 72(40.67%) | |
| Education | Illiterate | 168(52.83%) | 98(55.36%) |
| Literate | 150(47.16%) | 79(44.63%) | |
Biochemical parameters of moderate and severe cases of COVID-19 were tabulated in Table 2. It was observed that the values of CRP, Ferritin and LDH (< 0.001, < 0.05, < 0.001) significantly increased in severe cases of COVID-19 as compared to moderate cases of COVID-19 using independent t-test. On the other hand, liver enzymes ALT, AST, GGT and ALP were also markedly raised in severe cases as compared to moderate cases.
Table 2: Biochemical parameters in patients of group A (moderate infection) group B (severe infection of COVID-19).
| Biochemical Parameters | Group A, n=318
Mean±SD |
Group B, n=177
Mean±SD |
p-Value |
| C-reactive protein (CRP) (mg/dl) | 78.60+91.86 | 170.29+101.71 | < 0.001 |
| Ferritin (ng/ml) | 495.69+640.54 | 826.71+575.82 | < 0.05 |
| Lactate dehydrogenase (LDH) IU/L | 426.88+251.90 | 928+437.40 | < 0.001 |
| Bilirubin mg/dl | 0.77+1.32 | 1.95+1.48 | > 0.05 |
| Alanine aminotransferase (ALT) IU/L | 68.48+100.21 | 81.91+57.00 | > 0.05 |
| Aspartate aminotransferase (AST) IU/L | 58.69+91.18 | 73.71+112.80 | > 0.05 |
| Gamma-glutamyl transferase (GGT) IU/L | 58.33+94.65 | 76.49+48.81 | > 0.05 |
| Alkaline phosphatase (ALP) IU/L | 112.20+110.09 | 166.10+115.60 | > 0.05 |
p<0.05= significant independent t-test applied; p<0.001= highly significant
The Association of demographic characteristics and biomarkers with the severity of Covid-19 infection was determined by using multiple logistic regression in Table 3. Multiple regression showed that the odds of severity of Covid-19 infection among medical personnel was 2.26 times compared to non-medical personnel. LDH was more correlated (1.017) with severity of disease compared to Ferritin and CRP.
Table 3: Association of various parameters with the severity of COVID-19 infection, using multiple logistic regression analysis.
| Parameter | Cruder OR (95%CI) | Adjusted OR (95%CI) | |
| Age | 1.03(1.02-1.05) | 1.02(0.99-1.04) | |
| Occupation | Non- medical | REF | REF |
| Medical | 1.37(0.92-2.05) | 2.26(1.02-4.89) * | |
| C-reactive protein (CRP) | 1.00(1.00-1.01) | 1.00(0.99-1.00) | |
| Lactate dehydrogenase (LDH) | 1.01(1.01-1.02) | 1.01(1.01-1.03) * | |
| Ferritin | 1.00(1.01-1.00) | 1.00(0.99-1.01) | |
*p<0.05= Significant; REF: reference category
Based on age groups, it was shown (Figure 1) that 66.03% of patients age ranges between 25-55 years and 33.96% patients of age ranges between 56-75 years were in group A. 45.19% patients with age range between 25-55 years and 54.80% patients of age ranges between 56-75 years were in group B.
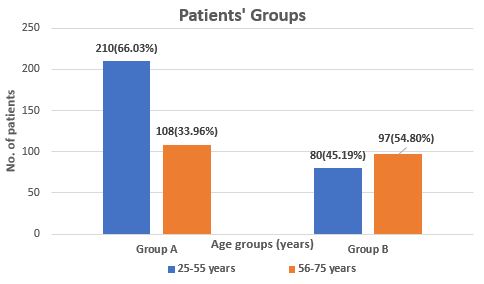
Figure: 1 Age groups of patients of group A (moderate infection) and group B (severe infection of COVID-19).
In the present study, we observed that males were more affected than females. It is stated that the immune system of females may have the ability to detect pathogen earlier than that of males due to their higher responses to antibody and higher immunoglobulin levels and number of B-cells11. The mean age of severe COVID-19 cases was 48.57 years and of moderate cases was 62.51 years. Among severe cases, 63% were male and 37% were female. It agrees with another study that observed comparable age in both genders and stated that the age of moderate to severe cases was in range of 50 to 75 years and more sufferers were male than female12,13.
The study proposed that older people are at more risk to develop this infectious disease as increasing age is directly related to general inflammation, which can impede the immune response. Thus, COVID-19 could push strongly against the overworked immune system. Besides, health states, health care schemes and co-morbidities, like obesity, diabetes and heart disease, also increase the infection fatality ratio14.
Patients associated with medical professions were more susceptible to COVID-19 than patients linked with non-medical profession. Studies have also reported morbidity and mortality in individuals associated with medical professions. The cause of COVID-19 infection in medical professionals is mainly due to physical closeness to patients and regular exposure to diseases15,16. As reported by Karlsson and Fraenkel that the risk of covid-19 infection in healthcare workers was three times more than non-healthcare workers. The risk was doubled among household members of front facing workers17.
Among biochemical parameters of mild and severe cases of COVID-19, it is observed that the values of CRP, Ferritin and LDH were significantly high in severe cases of COVID-19 as compared to moderate cases of COVID-19. The present study is in-line with several studies18. They also noted significantly high values of CRP and ferritin. One of the studies stated that the high values of CRP 19, and ferritin may be related to a secondary type of infection and indicate a poor prognosis of COVID-19. Another study found that high levels of both serum ferritin and CRP composite poor consequences. High values of serum ferritin were observed in older males20 and found to be independently related to acute respiratory distress syndrome and severity of COVID-19. A study speculated that COVID-19 induced synthesis of proinflammatory cytokines, might promote the synthesis of ferritin in the initial stages of inflammation that results in the damage of cells. The damage of cells may promote the seepage of ferritin from cells and increase its values in serum21. Similarly, a study observed that the gathering of Middle East Respiratory Syndrome coronavirus nanoparticles is associated with chaperone-arbitrated ferritin22.
It is proposed that increased levels of inflammatory cytokines and inflammatory biomarkers like CRP, interleukins 2, 6 and 7 and ferritin showed that extreme hyper inflammation may result in cardiopulmonary collapse and failure of a different organ like the liver23,24. Additionally, it is substantiated that the level of serum CRP may be used to monitor the treatment of patients infected with COVID-19. The present study consents with studies that observed significantly high levels of LDH. These studies stated that serum LDH is a useful marker for assessing the clinical severity of infection with COVID-19 and it helps to monitor the response of treatment in pneumonia associated with COVID infection. Therefore, it helps in the stratification of risk and primary intervention24. Additionally, a study reported that both CRP and LDH are associated with the function of lung/respiratory system and may be markers of respiratory system failure in patients with COVID-19 25.
Besides the significantly high levels of serum CRP, ferritin and LDH, we also observed markedly high values of liver enzymes ALT, AST, GGT and ALP in severe cases as compared to moderate cases. We support the studies those also observed high levels of serum CRP, Ferritin and LDH along with liver enzymes ALT, AST, GGT and ALP. A study stated that markedly high values of these parameters may indicate severity of COVID-19 26,27. A study reported that high levels of gamma-glutamyl transferase accompanied by aspartate aminotransferase indicate liver derangements in serious patients, while high values of lactate dehydrogenase indicate the risk of mortality. In a local study, findings were similar to our results that several markers including elevated leukocyte and neutrophil counts, CRP, LDH, and deranged liver enzymes were likely associated factors linked with disease severity and mortality28. In this retrospective analysis, not all biochemical parameters were monitored and liver derangements were not confirmed by the ultrasound.
Serum Ferritin, C-reactive protein and Lactate Dehydrogenase can be the prognostic biochemical parameters related to the severity of disease in patients with COVID-19. Furthermore, the increased values of liver enzyme indicate liver derangement in such patients.
We are thankful to the administration of hospital from where samples were collected for the present study.
There was no conflict of interest among the authors.
Verbal and written informed consents were obtained from all patients.
The ethical approval was taken from Research Review Committee of Post Graduate Medical Institute (ERC number: 00138-20).
WS was involved in the conception and data acquisition. KN and AJ were involved in literature search, analysis and interpretations. RK and SS participated in manuscript writing and proof reading
- Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):1-14.
- Schett G, Manger B, Simon D, Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol. 2020; 16:465-470.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
- Lin Z, Long F, Yang Y, Chen X, Xu L, Yang M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect. 2020;81(4):647-679.
- Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(4):332-334.
- Martinez-Outschoorn UE, Prisco M, Ertel A, Tsirigos A, Lin Z, Pavlides S, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle. 2011;10(8):1271-1286.
- Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38(9):1722-1726.
- Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8(1):13-17.
- Omrani-Nava V, Maleki I, Ahmadi A, Moosazadeh M, Hedayatizadeh-Omran A, Roozbeh F, et al. Evaluation of hepatic enzymes changes and association with prognosis in COVID-19 patients. Hepat Mon. 2020;20(4):1-6.
- Released by National Health Commission and National Administration of Traditional Chinese Medicine on March 3, 2020. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl). 2020;133(9):1087-1095.
- Abdullah M, Chai PS, Chong MY, Tohit ER, Ramasamy R, Pei CP, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272(2):214-219.
- Asghar MS, Kazmi SJ, Khan NA, Akram M, Hassan M, Rasheed U, et al. Poor prognostic biochemical markers predicting fatalities caused by COVID-19: a retrospective observational study from a developing country. Cureus. 2020;12(8):1-17.
- Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80(6):14-18.
- Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY). 2020; 12(10): 9959-9981.
- Higginson J, Walters R, Fulop N. Mortality and morbidity meetings: an untapped resource for improving the governance of patient safety? BMJ Qual Saf. 2012;21(7):576-585.
- Liu Q, Luo D, Haase JE, Guo Q, Wang XQ, Liu S, et al. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Glob Health. 2020;8(6):e790-e798.
- Karlsson U, Fraenkel CJ. Covid-19: risks to healthcare workers and their families. BMJ. 2020; 371-372.
- Manson JJ, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2(10): e594-e602.
- Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1-14.
- Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748-773.
- Kim YS, Son A, Kim J, Kwon SB, Kim MH, Kim P, et al. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front Immunol. 2018;9:1-20.
- Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020; 39(5): 405-407.
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin Immunol. 2020;214:1-6.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
- Wu MY, Yao L, Wang Y, Zhu XY, Wang XF, Tang PJ, et al. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir Res. 2020;21(1):1-6.
- Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, Dacrema A, et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta. 2020;509:135-138.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720.
- Asghar MS, Kazmi SJ, Khan NA, Akram M, Khan SA, Rasheed U, et al. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi. Cureus. 2020;12(6):1-29.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
