By Shahzad Ali Jiskani1, Halar Rahim2, Naila Noor3, Rab Nawaz Sathio4, Saba Ismaili Khawaja5, Rizwan Ali Talpur3
AFFILIATIONS:
- Department of Pathology, Indus Medical College, Tando Muhammad Khan.
- Department of Medicine, Jinnah Postgraduate Medical Centre, Karachi.
- Department of Physiology, Isra University, Hyderabad.
- District Headquarter Hospital, Tando Muhammad Khan.
- Department of Pathology, Government Hospital Qasimabad, Hyderabad, Pakistan.
ABSTRACT
Background: The morphology of megakaryocytes plays a major role in thrombopoiesis. Dysmegakaryocytopoiesis and thrombocytopenia can result from imperfection in any phase of megakaryocytopoiesis. The structure, morphology, and characteristics of megakaryocytes can differentiate and identify the etiology of underlying diseases such as leukaemia, mixed anaemia, idiopathic, aplasia, or infiltration. This research aimed to comprehend megakaryocyte attributions and their contribution to the diagnosis of thrombocytopenia.
Methods: This was a cross-sectional analysis of all successive cases of thrombocytopenia bone marrow aspirates over 12 months (January 2019 to December 2019) at the Department of Pathology, Indus Medical College Hospital Tando Muhammad Khan. With a 100X lens, megakaryocyte morphology was studied. Data were analysed by SPSS and Chi-square was performed to find correlation at the 95% confidence interval.
Results: Among the 91 subjects of thrombocytopenia, the commonest etiology of thrombocytopenia was idiopathic thrombocytopenia 34(37.36%). The common morphological changes in megakaryocytes were hypolobated megakaryocytes and bare megakaryocyte nuclei. Megakaryocyte numbers were increased for 31 (91.17%) patients among idiopathic thrombocytopenia, though the megakaryocyte numbers were decreased among 19 (90.47%) patients having acute leukaemia. A change to immature, young, less polyploid and bare nuclei forms was observed in immune thrombocytopenia. Immature types were seen in 15 cases, 14 cases of bare megakaryocytic nuclei, 15 cases of hypolobation and one case of micromegakaryocytes.
Conclusion: Many similarities among various haematological diseases were observed in megakaryocytic morphology. However, in megakaryocytic thrombocytopenia, high megakaryocyte counts and evidence of bare nuclei of megakaryocytes (p=0.02) and hypolobic types (p=0.03) proved important and play a very essential role in diagnosis.
Keywords: Megakaryocytes; Thrombocytopenia; Bone Marrow Aspirate; Diagnosis; Dysmegakaryopoiesis.
Corresponding Author:
Dr. Shahzad Ali Jiskani
Department of Pathology,
Indus Medical College,
Tando Muhammad Khan, Sindh, Pakistan.
Email: [email protected]
The megakaryocytes derive from the pluripotent hematopoietic stem cells, which go through the involvement of lineage, differentiation, and proliferation under the control of cytokines, specifically thrombopoietin (TPO) to produce 1000–3000 platelets1. This is evident that the morphology of megakaryocytes takes an essential part in thrombopoiesis and those additional platelets than smaller platelets are produced by giant megakaryocytes. The higher the platelet output, the greater the ploidy (nuclear lobulation) will happen. The processes that regulate the size of platelets are incompletely known but it appears that the body is trying to the preservation of total mass of the platelets and not a total count of the platelet. When multiplying the overall count of platelets by mean platelet volume, the total mass of platelets is constantly extracted1.
As the count of platelets reduces, mean platelet volume enhances. When the peripheral smear reveals megathrombocytes (giant platelets), it typically suggests peripheral platelet intake. Platelets play critical roles in daily life restoration of minute vascular damage, repair of the wound, the response of the innate immune system and biology of metastatic cell tumours. The mean number of platelets in humans varies from 150-350 x 109/L, although the amount is kept within reasonably narrow limits for any person. The replication of deoxyribonucleic acid (DNA) of megakaryocytes that happens devoid of cell division results in normal maturation and platelet formation, cause a giant lobulated polypoid nucleus. Wide range of growth factors, such as the thrombopoietin, function concurrently with other cytokines of haematopoiesis and factors of transcription that stimulate megakaryocyte growth and maturation. Dysmegakaryocytopoiesis and thrombocytopenia may lead to a fault in either phase of megakaryocytopoiesis2.
Thrombocytopenia is characterized as less than 1, 50, 000/mm3 of platelet counts. It is the source of bleeding that is most common. Thrombocytopenia arises from only four mechanisms, considering the amount and variety of disorders that are etiologically related: artifactual thrombocytopenia, low development of platelets, rapid degradation of platelets and an unequal distribution or platelet pooling inside the body3. Changes in the morphology of megakaryocytes in immune thrombocytopenic purpura, e.g., wide spread vacuolization of cytoplasm, hypogranularity and smoothing of the cell membrane, were previously identified by Frank in 1915and further verified by other researchers. This was claimed that certain changes were artefacts caused via techniques of staining or fixation1.
Changes of dysplasia in thrombocytopenia affiliated to a myelodysplastic syndrome in megakaryocytes are well documented. Non-myelodysplastic haematological disorders, such as immune thrombocytopenic purpura, aplastic anemia, hypersplenism, acute myeloid leukaemia, acute lymphoblastic leukaemia, leukaemia lymphoma syndrome, metastasis of bone marrow, blast crisis of chronic myeloid leukaemia is often found in megakaryocytes. However, there is no evidence on the occurrence of megakaryocyte dysplastic shifts in non-myelodysplastic haematological conditions2. Study in 1995 found that myelodysplastic syndrome is distinguished by the occurrence of atypical micromegakaryocytes4. By 2009, idiopathic thrombocytopenic purpura (ITP) was proved to have increased megakaryocyte number with immature forms2. Study in 2010 revealed megakaryocytes in megaloblastic anaemia were hyperlobated with finely stippled chromatin5. A 2012 study similarly postulated that immune-mediated thrombocytopenic purpura is distinguished via major morphological changes in bone marrow megakaryocytes6.
Additional researches on the assessment of megakaryocytic modification along with its relation with thrombocytopenia may give pathogenesis awareness regarding various haematopoietic problems that can recognise the wider clinical implementation of the recent strategies for controlling function and control of platelet. This research aimed to identify changes in the morphology and number of megakaryocytes among various haematological disorders that cause thrombocytopenia as well as determining if certain modifications had an important connection to various etiologies of thrombocytopenia.
It was cross-sectional research carried out at Indus Medical College Hospital, Tando Muhammad Khan, with individuals of thrombocytopenia undergone bone marrow aspirates for1 year period (January 2019 to December 2019). The study was conducted after approval from Ethical Board Review of the Institution (No.ERB/IMC/2018-026). The research included all participants with thrombocytopenia for which bone marrow aspiration was performed. Cases that met the thrombocytopenia but lacked a characteristic bone marrow aspirate were removed from the study. The subjects received informed consent and retained confidentiality during the study. Clinical information such as history, physical observations, peripheral blood smear, full blood counts and other necessary related parameter studies were assessed. The peripheral smears were organized and stained by using Giemsa stain as per standard guidelines7. Bone marrow aspiration was done from the posterior superior iliac spine in subjects having thrombocytopenia and then stained with Giemsa, followed by examination under the microscope. Rating of megakaryocytes was organized as normal megakaryocytes: one megakaryocyte/1-3 low-power fields, increased megakaryocytes: >two megakaryocytes/low-power field, or decreased megakaryocytes: one megakaryocyte/5-10 low-power fields1.
The characteristics of megakaryocytes were evaluated with microscopy (100x)8, including immature structure/form, segmentation of nucleus, dysplasia, micromegakaryocytes, budding of the platelets, vacuolization in the cytoplasm, bare nuclei of megakaryocyte, hypolobulation, and hypogranulation. Dysmegakaryopoiesis is the presence of irregular megakaryocytes comprising dysplasia, micromegakaryocytes, megakaryocytes having split lobes and hypogranulation. It is postulated that typical megakaryocytes have4-16 lobes of nucleus. Megakaryocytes with immaturity were classified as young megakaryocyte types. They usually have slight cytoplasm with a bluish colour and lack of lobes of the nucleus occupying most of the cell. Megakaryocytes having single or multiple distinct nuclei are known as dysplastic megakaryocytes. The micromegakaryocytes are classified as megakaryocytes with size as that of the single/bilobed nucleus of a large lymphocyte/ monocyte. If cytoplasmic processes emerge from their surfaces, megakaryocytes are supposed to exhibit budding of platelets. Megakaryocytes having water clear or pale grey cytoplasm and no or light granules are known as hypogranular types. It is also known as the form of cell observed in emperipolesis inside megakaryocyte. Morphology and the number of megakaryocytes with thrombocytopenia were subsequently evaluated.
The data collected were tested for totality, entered, and analyzed by SPSS. The statistics with descriptive nature were prepared using charts and statistical tests (e.g., Chi-squared test) were performed to find correlation of megakaryocyte morphology and various causes of thrombocytopenia at the 95% confidence interval.
Among all 91 bone marrow aspirate samples, thrombocytopenia was present in groups of all ages; though most subjects were younger than 30 years (64.83%). Similarly, females (56.04%) were more affected than males. The age was ranged between 6 to 50 years. A 48 (52.74%) were males while 43 (47.25%) were females, 64 (70.32%) were >12 years of age while 27 (29.67%) were <12 years of age. Bleeding tendency (n=31; 34.06%) followed by pallor (n=29; 31.86%) was commonest clinical characteristic observed. In acute leukaemia, the typical clinical characteristics were fever (92.3%), pallor (68.13%) and organomegaly (64.83%), commonest etiology of thrombocytopenia for which aspiration of bone marrow was pursued was idiopathic thrombocytopenia (37.36%), followed by acute leukaemia (24.17%), as shown in Figure 1.
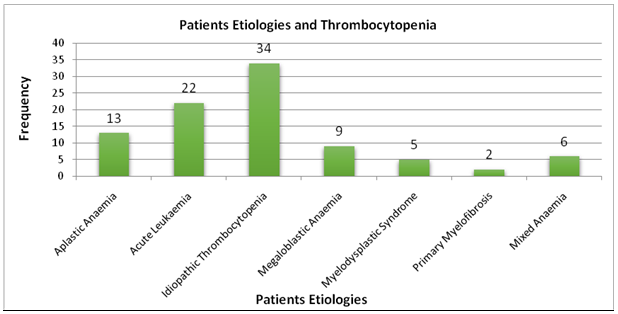
Figure 1: Etiologies in patients with thrombocytopenia.
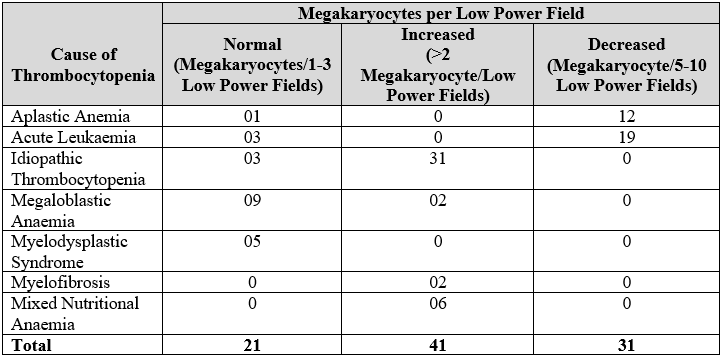
As shown in Table 1, megakaryocyte numbers were increased for 31patients (91.17%) among idiopathic thrombocytopenia, though the megakaryocyte numbers were decreased among19 patients (90.47%) having acute leukaemia. By observing the characteristic megakaryocyte morphology, particular features of morphology observed among all patients having idiopathic thrombocytopenia in the current research was a change to immature, young, less polyploid and bare nuclei forms. Immature types were seen in 15 cases, 14 cases of bare megakaryocytic nuclei, 15 cases of hypolobation and one case of micromegakaryocytes.
Table 1: Megakaryocyte among various etiologies of thrombocytopenia.
In Table 2, the morphology observed was tabulated. The chances of an increased idiopathic megakaryocyte count are higher than for other thrombocytopenia (Table 3).
Table 2: Megakaryocyte morphology as per etiologies of thrombocytopenia.
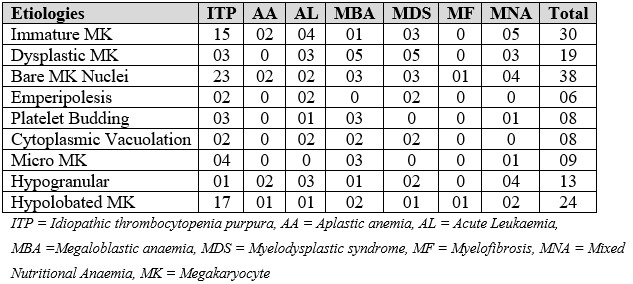
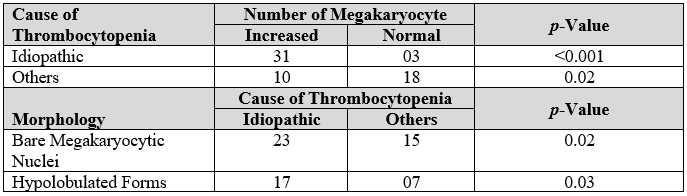
Table 3: Association of cause of thrombocytopenia and megakaryocytes.
In the current study, thrombocytopenia was observed with an average age of 27.18 years. ITP was the most common cause. Acute leukaemia accompanied by aplastic anaemia was the next commonest etiology; myelofibrosis and megaloblastic anaemia was the least common. The morphological features and number of megakaryocytes correlated to various patients of thrombocytopenia may be observed by Giemsa-stained smears of bone marrow aspirate. For a wide variety of haematological disorders, this may enhance diagnostic precision, thereby allowing adequate therapeutic strategies2.
Acute myeloid leukemia (AML) was commonest reason for thrombocytopenia. Idiopathic thrombocytopenia purpura (ITP) followed by dimorphic anaemia and acute lymphoblastic leukaemia (ALL) was next commonest cause2. Though in our study, ITP was the commonest cause followed by acute leukaemia. Megakaryocyte number was increased in our research, which was found by George et al.26 and Levine 27 as well. The relation was with activation of megakaryocytes in marrow to synthesise platelets at accelerated speed due to immune-mediated destruction of platelets9,10. Wang and Shen observed the thrombocytopenia severity with enhanced megakaryocyotpoiesis correlated to elevated mean platelet volume (MPV) among ITP patients9.
In study by Muhury et al., number of bone marrow megakaryocytes was elevated in 18 of 19 cases with ITP, like the current study. Glycoprotein Ib/IX and IIb/IIIa antiplatelet autoantibodies observed in ITP compromise the platelet development and liberation by triggering the degradation and abnormal maturation of megakaryocytes2,10,11. They found over-proliferation of bone marrow megakaryocytes (MKs) along with abnormal characteristics of megakaryocytes in most patients with ITP by Hu et al12. In the study by Dameshek and Miller, megakaryocytes were elevated in bone marrow of acute and chronic ITP, while circulating platelets in blood were uncommon, the finding analogous to current research13,28. Development of platelets was significantly increased following splenectomy, which was seen in 69-81%; huge masses of fresh marrow platelets were also very remarkable.
Results of an increase in megakaryocytes along with substantial decrease in production of platelets in marrow prior to splenectomy and a pronounced rise in manufacturing of platelets following splenectomy suggest explicit pathogenetic association between spleen and disease13. Characteristic morphology of megakaryocytes in ITP was similar with results by Nurden29, in which they said it is due to mature megakaryocytes’ para – apoptotic and apoptotic variety of programmed cell death. Improper programmed cell death of mature megakaryocytes may interrupt the production of platelets and ITP occurs with para-apoptosis form of programmed cell death. This result is particularly beneficial when isolated thrombocytopenia is present in some myelodysplastic syndrome (MDS) patients, thus mimicking ITP1.
Emperipolesis was found in a research performed by Rozman and Vives-Corrons among 1 of 17 patients of IP with lymphocytosis18. In our study, emperipolesis was observed in 2 cases of ITP. Levine27 and Houwerzijl et al. have observed vacuolization in cytoplasm in 1 case1,9. In our study, it was observed in 8 cases with majority of them having ITP and acute leukaemia. Autophagy to sustain cell metabolism is another possible reason for cytoplasmic vacuolization while metabolic demand is increased and nutrition deficiency is evident due to high megakaryocyotpoiesis.
In ITP, the existence of bare megakaryocytic nuclei was important. Likelihood of ITP was greater by bare nuclei of megakaryocytes than with other etiologies of thrombocytopenia. Bare nuclei of megakaryocytes were observed in 16 ITP patients (84.2%), in study conducted by Muhury et al 2. In this analysis, megakaryocytes turned absent or reduced among patients with aplastic anaemia that was seen by de Masson et al.30 as well. They related it to repression of the bone marrow as well as major inhibition in the megakaryocytes of synthesis of nucleic acid15.
Findings shown by Dameshek and colleague28 also demonstrated that number of lymphocytes enhanced and that of megakaryocytes decreased in bone marrow among patients with aplastic anaemia that revealed failure of hematopoietic function. However, no any morphological anomalies occurred among the patients of bone marrow of aplastic anaemia13,16,20. While young, naked, hypolobic and hypogranular structures were observed in present sample.
In aplastic anaemia, hypolobate, bare, immature, and dysplastic types with emperipolesis, vacuolization of cytoplasm, and budding of platelets of megakaryocytes were seen in research of Muhury et al.2,17,21, similar findings as in our study. Though, in aplastic anaemia, Tricot et al. showed regular morphology of megakaryocytes23,23. Similarly, not many cases of myelodysplastic syndrome, myelofibrosis, megaloblastic anaemia, and mixed nutritional anaemia along with idiopathic thrombocytopenia were included in this analysis for comparison. There were 5 MDS cases, all of which displayed a normal number of megakaryocytes in the bone marrow18,24,25. Discovery of emperipolesis among anaemia was observed in Tavassoli study19. In the current research, though, dysplastic megakaryocytes and emperipolesis were very few, which may be because of lesser sample size. For cases of thrombocytopenia because of myelodysplastic or other etiologies, morphological changes of megakaryocytes are valuable and carry particular attention. Therefore, current study postulates that diagnostic accuracy of various etiologies of thrombocytopenia is enhanced by associating the various types of megakaryocytes found with the number of megakaryocytes present in the aspiration slide of the bone marrow. Therefore, for an extensive range of haematological conditions, knowledge of changes of morphology of megakaryocytes can enhance the diagnostic accuracy.
There are many similarities between different haematological diseases according to the morphological changes of megakaryocytes. However, a diagnostic approach can diverge the available comprehensive information. But in this research, idiopathic thrombocytopenia enhanced the number and the existence of bare nuclei of megakaryocytes and hypolobulation which could be important for further investigations.
The authors acknowledge the institution and technical staff for their support.
The authors declare no conflict of interest.
The Ethical Review Board of the Institution had approved the study with the reference No.ERB/IMC/2018-026.
As this was laboratory-based study, patients were only informed verbally.
SAJ had given the study concept, performed experimentation, and written the discussion. HR performed the data analysis while NN interpreted the results. RNS had also contributed in the data analysis and written the literature review. SIK assisted in experimentation and RAT also helped in literature review writing.
- Houwerzijl EJ, Blom NR, van der Want JJ, Esselink MT, Koornstra JJ, Smit JW, et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood. 2004;103(2):500-506.
- Muhury M, Mathai AM, Rai S, Naik R, Pai MR, Sinha R. Megakaryocytic alterations in thrombocytopenia: A bone marrow aspiration study. Indian J Pathol Microbiol. 2009;52:490-494.
- Lev PR, Goette NP, Mara RF. Pathophysiological mechanisms leading to low platelet count in immune thrombocytopenia. J Immunological Sci. 2020;4(2):1-7.
- Zhu J, Wang B. Megakaryocytes in peripheral blood smears of non-hematological diseases. Int J Hematol. 2020;112:128-130.
- Chapman J, Geyer JT, Khanlari M, Moul A, Casas C, Connor ST, et al. Myeloid neoplasms with features intermediate between primary myelofibrosis and chronic myelomonocytic leukemia. Mod Pathol. 2018;31(3):429-441.
- Vinayakamurthy S, Potluri R, Shivajirao P, Singh R, Pujahari R, Maniketh I. A study of megakaryocyte morphology in bone marrow aspiration smears of cases of thrombocytopenia. Med J. 2017;10(1):51-57.
- Moreau T, Evans AL, Vasquez L, Tijssen MR, Yan Y, Trotter MW, et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun. 2016;7(1):1-6.
- Feng G, Gale RP, Cui W, Cai W, Huang G, Xu Z, et al. A systematic classification of megakaryocytic dysplasia and its impact on prognosis for patients with myelodysplastic syndromes. Exp Hematol Oncol. 2015;5(1):1-8.
- Wang ZY, Shen ZX. 5 Megakaryocytes and platelets in immune thrombocytopenic purpura. Baillieres Clin Haematol. 1997;10(1):89-107.
- Wang L, Li Y, Hou M. Idiopathic thrombocytopenic purpura and dysmegakaryocytopoiesis. Crit Rev Oncol Hematol. 2007;64(2):83-89.
- Raj AB. Immune Thrombocytopenia and Treatment Approaches. J Hematol Transfus. 2017;5(1): 1056-1064.
- Hu T, Shi XD, Feng YL, Liu R, Li JH, Chen J, et al. Comparative study on bone marrow megakaryocytes in children with thrombocytopenic purpura, aplastic anemia and myelodysplastic syndrome. Zhonghua Er Ke Za Zhi. 2005;43(3):183-187.
- Jawaid A. Haematological causes of thrombocytopenia in children at Aga Khan University Hospital, Karachi. J Pak Med Assoc. 2015;65(4):347-359.
- Suljević D, Filipić F, Islamagić E. Emperipolesis: Sternal and femoral microenvironment induces megakaryiocyte emperipolesis in the Wistar strain. Maced Vet Rev. 2019;42(1):71-77.
- Young NS. Aplastic Anemia. N Eng J Med. 2018;379(17):1643-1656.
- Tricot G, Vlietinck R, Boogaerts MA, Hendrickx B, Wolf‐Peeters CD, Van den Berghe H, et al. Prognostic factors in the myelodysplastic syndromes: importance of initial data on peripheral blood counts, bone marrow cytology, trephine biopsy and chromosomal analysis. Br J Haematol. 1985;60(1):19-32.
- Sun RJ, Shan NN. Megakaryocytic dysfunction in immune thrombocytopenia is linked to autophagy. Cancer Cell Int. 2019;19(1):1-10.
- Rozman C, Vives‐Corrons JL. On the alleged diagnostic significance of megakaryocytic ‘phagocytosis’(emperipolesis). Br J Haematol. 1981;48(3):510.
- Tavassoli M. Modulation of megakaryocyte emperipolesis by phlebotomy: megakaryocytes as a component of marrow-blood barrier. Blood Cells. 1986;12(1):205-216.
- Tang YT, He P, Li YZ, Chen HZ, Chang XL, Xie QD, et al. Diagnostic value of platelet indices and bone marrow megakaryocytic parameters in immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2017;28(1):83-90.
- Song Y, Shi MM, Zhang YY, Mo XD, Wang Y, Zhang XH, et al. Abnormalities of the bone marrow immune microenvironment in patients with prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(6):906-912.
- Iraqi M, Perdomo J, Yan F, Choi PY, Chong BH. Immune thrombocytopenia: antiplatelet autoantibodies inhibit proplatelet formation by megakaryocytes and impair platelet production in vitro. Haematol. 2015;100(5):623-632.
- Hicks SM, Coupland LA, Jahangiri A, Choi PY, Gardiner EE. Novel scientific approaches and future research directions in understanding ITP. Platelets. 2020;31(3):315-321.
- Soliman MA, Eldeen SM, Elhawy MA, Elshafey OH. Role of cellular immunity in the pathogenesis of immune thrombocytopenic purpura in children. Menoufia Med J. 2018;31(2):550-556.
- Lev PR, Goette NP, Marta RF. Pathophysiological mechanisms leading to low platelet count in immune thrombocytopenia. J Immunol Sci. 2020;4(2):1-7.
- George JN, El-Harake MA, Raskob GE. Chronic idiopathic thrombocytopenic purpura. N Engl J Med. 1994;331(18):1207-1211.
- Levine FC. Idiopathic thrombocytopenia. Arch Intern Med. 1999;88: 701-728.
- Dameshek W, Miller EB. The megakaryocytes in idiopathic thrombocytopenic purpura, a form of hypersplenism. Blood. 1946;1(1):27-51.
- Nurden AT. Qualitative disorders of platelets and megakaryocytes. J Thromb Haemost. 2005;3(8):1773-1782.
- de Masson A, Bouaziz JD, de Latour RP, Benhamou Y, Moluçon-Chabrot C, Bay JO, et al. Severe aplastic anemia associated with eosinophilic fasciitis: report of 4 cases and review of the literature. Med. 2013;92(2):69-81.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
