By Faraz Saleem1, Muhammad Owais Ismail1, Zahida Memon1, Kausar Aamir2, Akhtar Ali1, Muhammad Mohtasheemul Hassan3
AFFILIATIONS:
- Department of Pharmacology, Ziauddin University.
- Department of Pharmacology, Basic Medical Sciences Institute (BMSI), Jinnah Post Graduate Medical Center (JPMC).
- Department of Pharmacognosy, University of Karachi, Karachi, Pakistan.
ABSTRACT
Background: Depression is a far more devastating to healthy state than chronic physical illnesses. In many cases, diagnosis of depression is difficult which makes physicians unable to design rational therapeutic prescription. Coriandrum sativum (CS) has been investigated for gastrointestinal related disorders but its association with depression has not been fully explored. The study aimed to evaluate the antidepressant activity of Coriandrum sativum extract with fluoxetine drug in animal models of depression.
Methods: It was a preclinical study. Total 40 mice were divided into 6 groups i.e., Group 1 control (0.9% NaCl I.P), Group 2 Fluoxetine (0.5 mg/kg i.p.), Group 3, 4, 5 Coriandrum Sativum (1.6 mg/kg, 3.2 mg/kg, 6.4 mg/kg, respectively). The 3.2mg/kg of CS was the most effective immobility dose which was used for locomotor test. The locomotor test was performed on group 6 (10 mice) after induction of depression via forced swimming test to rule out central nervous system (CNS) stimulatory effect. ANOVA was applied for the Forced Swimming Test (FST) while paired t-test used for locomotor activity. p ≤0.05 was considered statistically significant.
Results: Fluoxetine and Coriandrum sativum showed statistically significant decrement (p=0.002) reduction in immobility time compared to control animals. The dose of 3.2 mg/kg, CS caused a highly significant reduction in immobility time (p-value 0.001), compared to fluoxetine. Pre- and post-analysis of locomotor activity in Group 6 did not exhibit any significant change in control counts (241.4) compared to test counts (241.5).
Conclusion: The diethyl ether extract of Coriandrum sativum was found to possess antidepressant activity similar to the standard prescription drug.
Keywords: Depression; Forced Swimming Test (FST); Coriandrum sativum (CS) Extract; Fluoxetine.
Depression remains a common psychological disorder that is very frequently seen in general medical settings and is expected to become widespread globally by 20301. It is described by persistent sadness and a lack of concentration in activities that a person normally appreciates, for at least two or more than two weeks. Depression often begins at a young age and affects females more often than males. It contributes a major fraction of a worldwide load of diseases and is one of the major causes of disability and premature death2. The World Health Organization (WHO) has graded depression as the 4th leading cause of debility globally3 and projects that by 2020, it will be the 2nd leading cause of inability4. According to WHO, Pakistan ranked 7th position with 7,436,224 reported cases of depression5. A study done by Mirza and his colleagues reported an overall 34% prevalence rate of depression in Pakistan6.
Depression results in a decrement in health state far more than the major chronic physical illnesses like angina, arthritis, asthma and diabetes7. In many cases, it becomes very difficult to diagnose depressed patients and consequently rational therapeutic prescription is not generated. Untreated depression leads to hippocampal volume loss, possibly resulting in increased stress sensitivity8 and increased risk of recurrence9. There has been remarkable progress in the treatment of depression. Multiple effective psychopharmacological interventions and psychotherapeutic treatment are in current use10. Most of the medicines that are selling today for depression are directly towards the modulating area of brain monoamine neurotransmission. The initial mechanism of the following medicines is increasing the concentration synaptic of monoamines like dopamine, serotonin along norepinephrine11. Among these drugs Selective Serotonin Reuptake Inhibitor (SSRIs), Fluoxetine is the most frequently used drug in a clinical setting.
Currently, there is a revival of interest in phytomedicine and herbs are playing a vital role in various diseases including depression. According to a WHO report, around 60% of the world population relies on some forms of traditional medicines mainly the herbs12. Coriandrum Sativum is one of the oldest herbs, belongs to family Apiaceae that has been used for over 3,000 years for both culinary and medicinal purposes13. Traditionally, coriander has been used to treat gastrointestinal disorders such as anorexia, dyspepsia, flatulence, diarrhea, pain and vomiting14. Coriander has long been used in Iranian traditional medicine as anticonvulsant, anti-depressant, sedative and anxiolytic agent15. Coriander extract inhibited Monoamine oxidases (MAO) activity, in a study the aqueous extract of seeds of Coriandrum Sativum showed marked decreases MAO-B activity with time, which was suggested to be the principal mechanism of anti-depressant-like activity16. Therefore, our study aimed to evaluate and compare the antidepressant activity of Coriandrum sativum extract with fluoxetine in animal models of depression.
It was a preclinical experimental animal study conducted at Ziauddin University Karachi from November 2019 to February 2020 after taken the approval from the animal ethics committee (Ref NO:2019-001). The 40 male BALB/c mice weighing 20-30gm were used in the study. Depression model was induced by performing Forced swimming test and for validation for antidepressant effect open field test was performed. The drugs were administered into the animals according to the following procedure.
Animals were kept under standard conditions with a normal light cycle (12 hours light/ dark) with free access to food and water. Mice were housed in plastic cages in groups of 6 animals per cage and wooden chips were used as bedding material. Prior to the experiment, animals were acclimatized with the experimenter and the environment for few days. All animals were dealt according to International Standards for the Use and Care of Laboratory Animals set by the National Institute of Health (US)17.
The animal grouping was done as follows: Group 1 (control) was administered with 0.9% NaCl intraperitoneally (I.P.); Group 2 was administered fluoxetine 0.5 mg/kg i.p., while Group 3, 4 and 5 were administered Coriandrum sativum at the dose of 1.6, 3.2 and 6.4 mg/kg I.P respectively. Group 6 was given the most effective dose of Coriandrum sativum for a locomotor test. The numbers of animals used in Groups 1 to 5 were 6 and 10 in Group 6.
Seeds of Coriandrum Sativum were procured from the local market of Karachi. The sample of the plant specimen was identified by the Department of Pharmacognosy, University of Karachi and allocated a voucher specimen no CSF-01-18/19. The seeds were washed; shades dried and were ground in a mixer. The powdered material was macerated with Diethyl ether for 14 days. The solvent was evaporated in a rotary evaporator with a water bath temperature of 40oC to get the extract. These crude extracts were placed openly in a ventilated room for 6-7 days to get dry and free from solvent completely. Dried extracts from seeds were packed in glass bottles with proper labeling. The extract then was stored in a refrigerator at 4°C until use18. Coriandrum sativum extract was emulsified in a control vehicle with 10% Dimethyl sulfoxide (DMSO) for intraperitoneal administration (i.p.0.2 mL/20g, mice).
Forced swimming test aimed to evaluate a hopeless state in rodents by forcing the animal to swim in a water cylinder without a method of escape. After an initial period of motor agitation, the animals naturally tend to adopt a motionless posture, performing movements only to keep their heads out of the water, indicating the animal had learned that escape is impossible. This behavior can be interpreted as if the animals had lost hope in this stressful situation19. Mice were placed individually in a glass tank (Height = 45 cm and Width =17 cm) filled with water to a height of 15 cm, and the temperature was maintained at 25°C. Each session was of 6 minutes duration, divided into pretest (the first 2 min) and test (the remaining 4 min). Vehicle control 0.9% NaCl i.p.in Group 1 and fluoxetine (0.5mg/kg i.p.) was administered in Group 2. Diethyl ether extract of Coriandrum sativum (1.6mg/kg, 3.2mg/kg, and 6.4mg/kg i.p.) was administered in Group 3, 4 and 5 respectively. After 1 hour of the treatment, mice were forced to swim under similar conditions as described above. The duration of immobility time was recorded for 4 minutes and the animal was considered immobile when it remained floating with all four limbs motionless20, 21.
The open-field test consists of an arena surrounded by high walls, to prevent escape, and the floor of the open field is divided into squares. In the test session, the number of square crossings with all four legs and time spent moving are used to assess the activity of the rodent. Crossings refer to the total number of square crossings during the test period, used to provide a measurement of locomotor activity of the animals19. Fifteen minutes before the observations mice were acclimatized to the open field apparatus. One hour before procedure vehicle control (0.9% NaCl or 10% DMSO solution i.p.) was administered and then ten minutes of locomotor counts were noted and the mean was calculated for Group 6. After 24 hours, the same animals received diethyl ether extract of Coriandrum sativum (most effective dose) at the same time under similar conditions and locomotor counts were recorded as described above (test counts). Test counts were compared with their respective control22. The results were analyzed by SPSS version 20, ANOVA test was used for determining the significance for inter and intra-group comparison and paired t-test was applied for the locomotor test.
In Table 1 Group 2 (Fluoxetine) and Coriandrum sativum groups that were3, 4 and 5 showed a reduction in immobility time as compared to group 1 (Control). Significant results (p-value=0.002) were observed with the fluoxetine group compared to the control. Group 4 Coriandrum sativum at the dose of 3.2 mg/kg also showed a significant reduction in immobility time (p-value= 0.001) compared to control (Table 1). When fluoxetine group was compared with CS groups (Group 3, 4 and 5) at different doses, we did not find any significant change (p≥ 0.05).
Table 1: Mean ± SD of test compounds after forced swimming test.
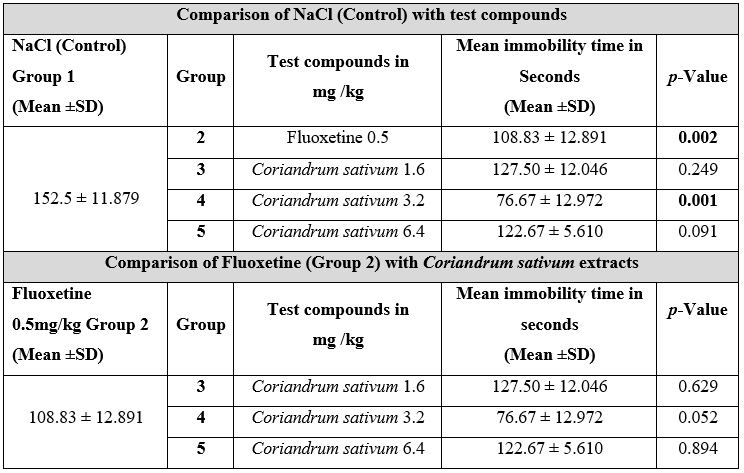
The intra-group comparison of CS at different doses showed that the most effective dose in our study was 3.2 mg/kg as shown in Figure 1(a). Pre- and post-analysis of locomotor activity in Group 6 did not exhibit any significant change in control counts when compared to test counts, which were counted at the most effective dose (3.2 mg/kg) of CS as shown in Figure 1(b).
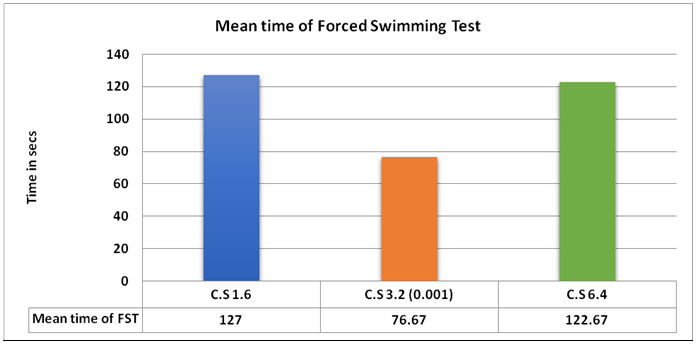
Figure 1(a): Intra Group comparison of Coriandrum sativum at different doses.
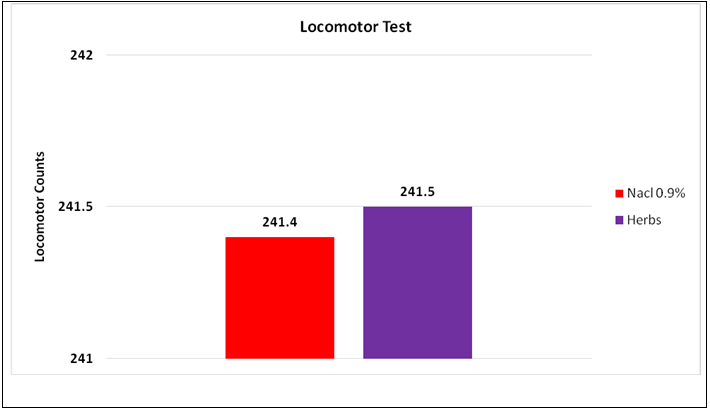
Figure 1 (b): Pre and post comparison of locomotor activity.
In the present study selective serotonin reuptake inhibitor, fluoxetine was used as a reference drug and the result was compared with the extract of Coriandrum sativum. Antidepressant properties were scientifically validated by conducting behavioral studies i.e. forced swimming test and locomotor test23.
In our study, fluoxetine caused a reduction in immobility time in mice after forced swim test (FST), which was statistically significant and these findings are similar to the previous findings of Porsolt et al. who created and validated the FST model by other antidepressant drugs such as tricyclic antidepressants, monoamine oxidase inhibitors and atypical antidepressants24. Fluoxetine caused a significant reduction in immobility time of mice in FST at 0.5 mg /kg, i.p. administration with a mean difference of 43.667 seconds and this result was similar with previous studies reported by Ismail et al. in 2009 and 201025,26. Almeida et al. in 2015 conducted a study by using rats in FST and administered fluoxetine 10 mg/kg, i.p. and the results seemed to be similar to our findings27. Parallel to our study Luo et al. reported that fluoxetine showed a reduction in immobility time in FST model at the dose of 0.67 mg/kg when administered via gastric intubation28.
The extract of Coriandrum sativum (1.3 mg/kg, 3.2 mg/kg and 6.4mg/kg respectively) showed a reduction in immobility time however, Coriandrum sativum at the dose of 3.2 mg/kg was found to have effective antidepressant activity in our study by using FST and showed a significant reduction in immobility time in mice as compared to control (Group 1). A study performed in India by Kharade et al. in 2011 reported similar results with a reduction in mobility time in mice using FST with I.P administration of diethyl ether extract of Coriandrum sativum at 2 mg/kg and 4 mg/kg respectively and aqueous extract of 200 mg/kg and 400 mg/kg orally16. Pathan et al. reported the reduction in immobility time in mice using FST but at different doses 100 mg/kg and 200mg/kg of ethanolic extract of Coriandrum sativum29.
When the doses (1.6 mg/kg, 3.2 mg/kg and 6.4 mg/kg respectively) of Coriandrum sativum were compared, it was observed that the 3.2 mg/kg dose caused maximum reduction in immobility time it was calculated to be statistically significant. Interestingly the antidepressant effect in our study seemed to be dose-independent and this finding was contrary to other studies that showed that the antidepressant effect was in dose dependent manner29. The possible reason for this conflicting finding may be hypothetically explained either by any unpredictable interactions amongst herbal constituents present in extract or variation in the concentration of agonist or antagonist component(s) of the extract. To the best of our knowledge and based on an extensive literature review, no report justified scientifically the reason behind this dose independent effect exhibited in our study.
In order to prove that the reduction in immobility time in forced swimming test is not caused by the possible central nervous stimulating effect, the most effective dose of Coriandrum sativum was investigated in the open field test / locomotor test. The extract of Coriandrum sativum did not cause any significant change in motor activity at the dose at which it produced a statistically significant reduction in immobility time of animals in a forced swimming test. These findings were similar to other antidepressants29. Pre and post analysis of locomotor activity with the most effective dose of plant extract did not show any statistically significant change in the motor counts when compared to control, indicating that the reduction in immobility time was not based on any central nervous system stimulation but it also seconds the exhibition of antidepressant effect of this herb.
The diethyl ether extract of Coriandrum sativum at the dose of 3.2 mg/kg possesses antidepressant activity. The extract of Coriandrum sativum may found comparable to that of fluoxetine.
The authors acknowledge the Ziauddin University (Clifton Campus) for facilitating the study.
The authors declare no conflict of interest.
The Animal Ethics Committee of Ziauddin University had approved the study with the protocol number: 2019-001.
This research work was carried out in collaboration among all authors. MOI and FS developed the concept of study. FS performed experimental work, drafted the document and interpreted the results. MOI critically reviewed the article and finalized the results. ZM finally reviewed and approved. Herbal extraction and animal house facility were provided by MMH and KA respectively. AA performed data analysis. All authors read and approved the final manuscript.
- Khandaker GM, Zuber V, Rees JM, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. 2020;25(7):1477-1486.
- World Health Organization. Depression other common mental disorders: global health estimates [Internet]. World Health Organization; 2017 [cited 2020 Dec 21]. 1-24 p. Available from:https://www.who.int/mental_health/management/depression/prevalence_global_health_estimates/en/
- Hagar M, Roman G, Eitan O, Noam BY, Abrham Z, Benjamin S. A tellurium-based small immunomodulatory molecule ameliorates depression-like behavior in two distinct rat models. Neuromolecular Med. 2020;22(3):437-446.
- Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-138.
- Reynolds 3rd CF, Patel V. Screening for depression: the global mental health context. World Psychiatry. 2017;16(3):316.
- Mirza I, Jenkins R. Risk factors, prevalence, and treatment of anxiety and depressive disorders in Pakistan: systematic review. BMJ. 2004;328(7443):794-798.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27(23):6313-6319.
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65(10):1156-1165.
- Freeman MP, Fava M, Lake J, Trivedi MH, Wisner KL, Mischoulon D. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association Task Force report. J Clin Psychiatry. 2010;71(6):669-681.
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4(5):409-418.
- Louw G, Duvenhage A. The traditional health practitioners act (No 22 of 2007): A South African Constitutional mishap? 2016. J Cult Stud; 36(10):2.
- Ishikawa T, Kondo K, Kitajima J. Water-soluble constituents of coriander. Chem Pharm Bull. 2003;51(1):32-39.
- Bashir S, Safdar A. Science of Spices and Culinary Herbs [Internet]. 2nd Bentham Science Publishers; 2020. Chapter 3, Coriander seeds: ethno-medicinal, phytochemical and pharmacological profile; [cited 2020 Dec 7]. p. 39-64.
- Yasir M, Khanam T, Hafeel M. A review on Kishneez (Coriander sativum Linn.): A potential herb. Pharma Innov J. 2019; 8(9):503-506.
- Kharade SM, Gumate DS, Patil VM, Kokane SP, Naikwade NS. Behavioral and biochemical studies of seeds of Coriandrum sativum in various stress models of depression. Int J Curr Res Rev. 2011;3:4-11.
- Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine [Internet]. 2nd Academic Press; 2002. 15 p.
- Shukla P, Sharma A. Effect of some medicinal plants on growth of Mycobacterium tuberculosis, multi drug resistant Mycobacterium tuberculosis and Mycobacterium other than tuberculosis. J Microbiol Biotechnol Food Sci 2021;2021:199-201.
- Valvassori SS, Varela RB, Quevedo J. Animal Models for the Study of Human Disease [Internet]. 2nd Academic Press; 2017. Chapter 38, Animal models of mood disorders: focus on bipolar disorder and depression; [cited 2020 Dec 10]. p. 991-1001. Available from: https://www.sciencedirect.com/science/article/pii/B9780128094686000383
- Porsolt RD, Bertin A, Jalfre MJ. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327-336.
- Porsolt RD, Le Pichon M, Jalfre ML. Depression: a new animal model sensitive to antidepressant treatments. Nat. 1977;266(5604):730-732.
- Ismail MO, Dar A, Faizi S, Abidi L. Antidepressant like actions of Opuntia dillenii butanol fractions in rodents. Pak J Pharmacol. 2010;27(2):9-14.
- McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87(1):162-171.
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. EurJ Pharmacol. 1978;47(4):379-391.
- Ismail MO, Dar A. Comparison of the efficacy of fluoxetine, phenelzine and moclobemide in rodents using animal models of depression. Pak J Pharmacol. 2009;26(2):19-23.
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23(5):238-245.
- Almeida J, Duarte JO, Oliveira LA, Crestani CC. Effects of nitric oxide synthesis inhibitor or fluoxetine treatment on depression-like state and cardiovascular changes induced by chronic variable stress in rats. Stress. 2015;18(4):462-474.
- Luo L, Wang JN, Kong LD, Jiang QG, Tan RX. Antidepressant effects of Banxia Houpu decoction, a traditional Chinese medicinal empirical formula. J Ethnopharmacol. 2000;73(1-2):277-281.
- Pathan A, Alshahrani A, Al-Marshad F. Neurological assessment of seeds of Coriandrum sativum by using antidepressant and anxiolytic like activity on albino mice. Inventi Impact: Ethnopharmacol. 2015;3:102-105.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
