By Humera Rashid1, Fariha Ibrahim2, Mehwish Faiz2, Qamar un Nisa Naz1, Asma Ansari3, Faria Fatima1
- College of Medical Technology, Ziauddin University, Karachi, Pakistan
- Biomedical Engineering Department, Ziauddin University, Karachi, Pakistan
- A. Q. Khan Institute of Biotechnology and Genetic Engineering, University of Karachi, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD13-2/007
How to cite: Rashid H, Ibrahim F, Faiz M, Naz QN, Ansari A, Fatima F. The Effect of Curcuma Longa on Respiratory Tract Isolates: A Potential Natural Therapeutic Agent. Pak J Med Dent. 2024;13(2): 40-47. Doi: 10.36283/PJMD13-2/007
Background: Extensive use of antibiotics has led to emergence of antimicrobial resistance (AMR), hence, challenging the treatment of clinical bacterial isolates. Herbal agents like Curcuma longa (Turmeric) have gained prominence for their wide-ranging effects. This study aimed to identify antibacterial and cytotoxic potential of pure and processed Curcuma longa on respiratory tract bacterial isolates to develop an effective and sustainable treatment option.
Methods: This study was conducted from March to October 2023 at Medical Technology Laboratory, Ziauddin University. The antibacterial activity of pure and processed Curcuma longa ethanolic extracts against Moraxella catarrhalis, Klebsiella pneumoniae, Streptococcus pyogenes, and Haemophilus influenzae was checked by agar well diffusion method. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined through microdilution method. Phytochemical composition was qualitatively analyzed and cytotoxic potentials of Curcuma longa were tested through MTS assay. Mean values of turmeric extracts were compared by student’s t-test through SPSS version 17. p-value<0.05 is considered significant.
Results: Comparable antibacterial activity was observed for pure and processed Curcuma longa extract against tested bacterial organisms. Among them, Moraxella catarrhalis showed maximum zones of inhibition (20mm) when treated with pure Curcuma longa extract. Furthermore, MIC was achieved at 25mg/ml and 50mg/ml against tested isolates with the bactericidal mode of action. Enhanced phytochemical composition and 98% cell viability against NIH 3T3 mouse fibroblast cell lines were observed for pure Curcuma longa.
Conclusion: This study revealed effective antibacterial potential of Curcuma longa and prefers the use of pure Curcuma longa due to its better phytochemical composition and safety profile for cellular systems.
Keywords: Curcuma longa, Respiratory tract infections, Anti-bacterial agents, Cytotoxicity.
Antimicrobial Resistance (AMR) has emerged as a critical global health crisis, threatening the effectiveness of modern medicine against infectious diseases. Pakistan ranks 176th in AMR-related mortality with 4.95 million deaths in 2019 attributed to drug-resistant infections. One in five of these fatalities occurred in children under five years and the second leading cause of child mortality1-4.
Respiratory tract infections (RTIs), encompassing Pharyngitis, Nasopharyngitis, Tonsillitis, Otitis media, and Sinusitis, affect individuals of all age groups. Bacterial strains majorly responsible for these infections are Haemophilus influenzae, Streptococcus pyogenes, Moraxella catarrhalis, Staphylococcus aureus, Klebsiella species, and Streptococcus pneumoniae5, 6. The misuse of antibiotics in RTI treatment significantly contributes to bacterial resistance against them7. Bacteria exhibit multiple resistance mechanisms against antibiotics, restricting from either a lack of antibacterial selection pressure or the absence of the antibiotic target 8. Compounds extracted from natural plant sources have proved to be the safest option with minimal side effects 9, 10.
Curcuma longa, commonly known as turmeric belongs to the family Zingiberaceae. It is widely used in the food industries, as a coloring agent as well as an additive to impart flavor in curries. Curcuma longa has a wide antiquity of use due to its spectacular antimicrobial and antifungal activities; which is used in the treatment of a variety of diseases. Curcumin a phenolic compound derived from Curcuma longa (Turmeric), emerges as a promising natural bioactive compound with diverse medicinal properties. Studies support its effectiveness as a prebiotic, probiotic, antioxidant, antimicrobial, and cytotoxic agent, making it valuable in both the food and pharmaceutical industries. It is soluble in various organic solvents with variable absorption potential. Ethanol has proved to enhance the solubility, bioavailability, and antimicrobial potential of Curcuma longa specifically in RTIs 11-13.
This study aimed to explore the antibacterial role of Curcuma longa (Turmeric), as a pharmaceutical agent against fastidious respiratory tract bacterial isolates to develop an effective and sustainable treatment option.
This study was conducted from March to October 2023 at the Medical Technology Laboratory, Ziauddin University Karachi. The Curcuma longa (Turmeric) samples used in this study were obtained from commercially processed and heat-dried rhizomes of turmeric purchased from a local market. All four clinical isolates i.e., Streptococcus pyogenes, Moraxella catarrhalis, Haemophilus influenzae, and Klebsiella pneumoniae were obtained from the Clinical Microbiology diagnostic laboratory of a Ziauddin Hospital North Nazimabad and sub-cultured on Sheep Blood agar (Oxoid CM0271, UK) and Chocolate agar (Oxoid CM0271, UK). Inoculated plates were incubated in 5% CO2 at 37˚C aerobically. The plates were examined after 24 hours for bacterial growth.
Samples of Curcuma longa (processed and pure rhizome) were extracted in ethanol. The Curcuma longa rhizomes were washed, ground with an electric grinder, and dried at 100°C for 3 hours. Samples weighing 50gm were dissolved in 500ml of 95% ethanol, and retained for 24 hours. The concentrated and filtered samples were then kept at 4°C.
As per Clinical & Laboratory Standards Institute (CLSI) guidelines 5% Sheep Blood agar for Streptococcus pyogenes, Haemophilus testing media (HTM) (Oxoid CM0898B, UK) agar for Haemophilus influenzae, and Muller Hinton (MH) (Oxoid CM0337B, UK) agar for Moraxella catarrhalis and Klebsiella pneumoniae were used to evaluate antimicrobial effect of Curcuma longa in pure and processed ethanolic extract through Agar well diffusion method hollowed by 6mm sterile borer 14, 15.
The stock was prepared by dissolving 100mg extract in 5 ml of dimethyl sulphoxide (DMSO) (Anala R®, BDH Laboratory Supplies England). From this stock solution, three working concentrations were prepared as 100mg/ml, 50mg/ml, and 25mg/ml (100μl in each well) with DMSO as a negative control. The plates were left for 15 minutes to allow for pre-diffusion and incubated at 37°C overnight (in triplicates). The zones of inhibition were measured after incubation; up to 9mm (inactive); 9-12mm (partially active), 13-18mm (active), and >18mm (active) 16.
Minimum inhibitory concentration (MIC) tested by Microtitre plate dilution. 50μl of bacterial suspension was prepared and added into the wells of a sterile 96-well microtitre plate. Each well of plate previously contained different concentrations of Curcuma longa extracts 100mg/ml, 50mg/ml, 25mg/ml, 12.5mg/ml, 6.25 mg/ml, and 3.12 mg/ml. Control wells contained culture medium and extract. The plate was incubated for 24 hours at 37°C. The point at which bacterial growth was completely inhibited, was determined to be MIC 17,18. Minimum bactericidal/bacteriostatic (MBC) was determined as 50μl from all wells spread on culture plates and incubated for 24 hours at 37°C 19.
Qualitative Phytochemical analysis of all the major components (Flavonoids Alkaloids, Proteins and Amino acids, Carbohydrates, starch, Phenol, Terpenes, Resins, Saponins, Phlobatannins, Tannins, and Glycosides) of pure and commercially processed Curcuma longa was done through reported methods 20-24.
Cytotoxic potential of pure and processed Curcuma longa extracts was qualitatively analyzed through 3-(4, 5 dimethyliazol-2-y1) 5 (3 carboxymethoxyphenyl) 2 (4 sulfophenyl) 2H tetrazolium (MTS) assay. Cells were cultured and 5 x 103 cells/well were added onto 96 well plates in triplicates. Plates were incubated for 24 hours at 5% CO2. Cell viability and proliferation of NIH 3T3 (mouse fibroblast cell line) were determined by exposing the viable cell to the Curcuma longa extracts in the presence of 5% CO2 at 37° C. After 24 hours, 20µL MTS solution was added in each well with further incubation for 2 hours. Growth was analyzed by reading the absorbance at 490 nm 25.
Obtained data (runs in triplicate) were compiled and tabulated. Mean values of pure and processed turmeric extracts in the targeted clinical isolates were compared by student’s t-test through SPSS version 17.0 (IBM, SPSS Inc.). P value < 0.05 is considered as significant.
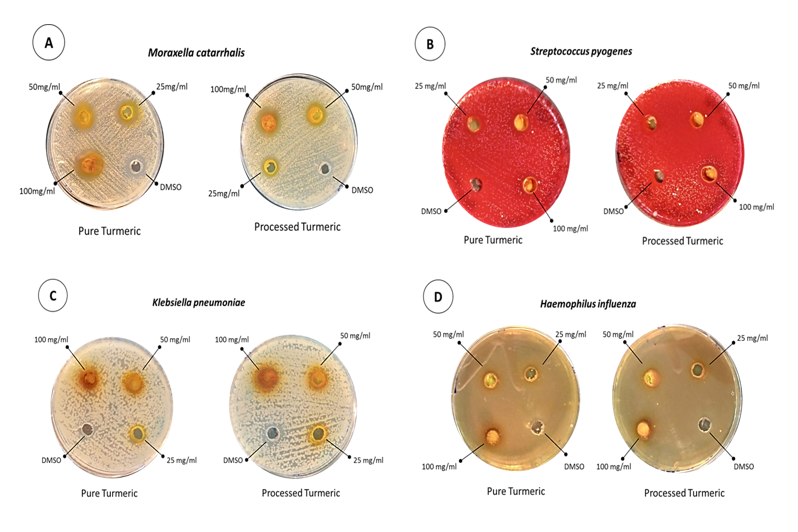
The antibacterial activity of Curcuma longa (Turmeric) was assessed against selected respiratory tract isolates; Moraxella catarrhalis, Streptococcus pyogenes, Klebsiella pneumoniae, and Haemophilus influenzae through the Agar well method. Stock solutions were prepared in DMSO, to rule out the bactericidal/ static effect of DMSO, it was used as a negative control 26. As shown in Figure 1 and Table 1 Moraxella catarrhalis, Streptococcus pyogenes, and Haemophilus influenzae were found to be sensitive whereas, Klebsiella pneumoniae has shown resistance against Curcuma longa extracts.
Figure 1: Antibacterial Activity of Pure and Processed Curcuma longa (Turmeric) represented as Zone of inhibition a) Moraxella catarrhalis; b) Streptococcus pyogenes; c) Klebsiella pneumoniae and d) Haemophilus influenzae
To determine the MIC microtiter plate method was used. Results showed that Moraxella catarrhalis demonstrated significant activity at low doses while Streptococcus pyogenes and Haemophilus influenzae showed moderate activity (Table 2). Klebsiella pneumonia showing resistance had not been tested for MIC. Furthermore, for Minimum bactericidal concentration (MBC) was monitored and the bactericidal effect of Curcuma longa was observed after incubation for tested isolates.
Table 2: Minimum Inhibitory Concentration (MIC) of Respiratory Tract Isolates.

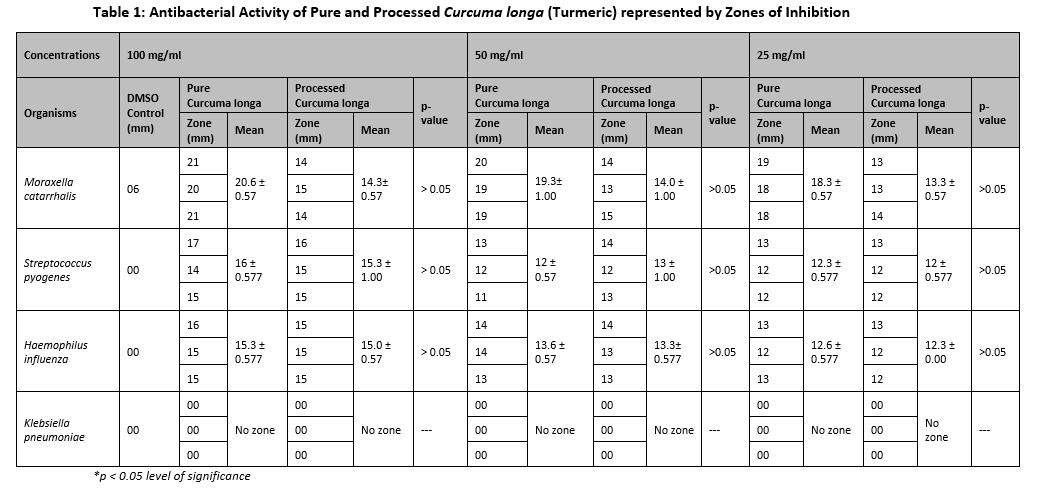
Qualitative analysis of the phytochemicals of both pure and processed Curcuma longa extracts revealed that the components in pure Curcuma longa are more profoundly present than processed ones (Table 3). Processing and packaging procedures may damage the phytochemical components of the processed Curcuma longa.
Table 3: Qualitative Analysis of the Phytochemical Constituents of Pure and Processed Curcuma longa.
Moreover, the Cytotoxic analysis of pure and processed Curcuma longa expresses great variability in results; on higher concentrations of 100mg/dl, both results were not readable due to color concentration. Cell treated with pure Curcuma longa showed 88% viability at 50 mg/ml and 98% at 25 mg/ml respectively. While, in the case of processed Curcuma longa the cell viability was reduced by 35%, and 60% viability was observed at 50mg/dl and 25mg/dl respectively, as depicted in Figure 2.

Figure 2: Cytotoxic Analysis of Pure and Processed Curcuma longa at different concentrations.
Curcuma longa (Turmeric) is traditionally an active agent due to its diverse therapeutic attributes. The complex interactions of this compound with specific pathogenic bacterial strains demand careful investigation 12, 27. The present study was focused on evaluating the antibacterial activity of Curcuma longa against Moraxella catarrhalis, Haemophilus influenzae, Streptococcus pyogenes and Klebsiella pneumoniae commonly causing respiratory tract infections. Besides the antibacterial activity, this investigation explores Cytotoxicity and Phytochemical composition of Curcuma longa.
The findings of antibacterial testing were comparable for pure and processed Curcuma longa. The largest zone of inhibition was formed by Moraxella catarrhalis followed by Streptococcus pyogenes, and Haemophilus influenzae whereas, no zone was formed by Klebsiella pneumoniae conferring resistance against both types of Curcuma longa extract as shown in Table 1. For sensitive strains zone formation larger than 12 mm was considered as an initiation value for active compounds28. Several studies have demonstrated the significantly higher activity of Curcuma longa against Gram-positive bacteria; as compared to Gram-negative bacteria. The difference in responsiveness is mainly based on the difference in the structure and components of bacterial cell walls 29, 30. However, interesting variations in sensitivity patterns emerged among the tested Gram-negative bacteria in this study; Moraxella catarrhalis displayed an enhanced response, while Haemophilus influenzae demonstrated a moderate reaction. Whereas, Klebsiella pneumoniae exhibited resistance. These findings aligned with prior research indicating that Curcuma longa’s effectiveness varies depending on the specific microbial species and strain. Notably, the sensitivity of bacterial species appears to be independent of their genus classification, highlighting a significant diversity in response to Curcuma longa 31.
Both extracts were further evaluated for MIC at varying concentrations from 100mg/ml (1:2) to 3.12mg/ml (1:64). Moraxella catarrhalis showed the lowest MIC values (25mg/ml) while Haemophilus influenzae and Streptococcus pyogenes displayed moderate sensitivity against Curcumin longa. These findings were concordant with the research conducted on the effect of Curcumin on respiratory tract isolates depicting the moderate activity against Haemophilus influenzae and Streptococcus pyogenes 32.
Furthermore, in the current study phytochemical composition of Curcuma longa (Turmeric) was analyzed as shown in Table 3. The chief biologically active component of Curcuma longa is Curcumin, which is a yellow phytochemical, hydrophobic, and polyphenol compound 33. General Glycosides, Carbohydrates, Starch, Flavonoids, Alkaloids, Phenols, and Proteins were present in both pure and processed Curcuma longa. Tannins, Terpenes, and Resins were present in pure Curcuma longa. While Saponins and Phlobatannins were absent in both pure and processed Curcuma longa. The presence of general Glycosides, Carbohydrates, Starch, Flavonoids, Alkaloids, Phenols, and Proteins in both the pure and processed Curcuma Longa highlights the healthy and constant composition. Glycosides, Carbohydrates, and Starch offer nutritive value, while Flavonoids and Phenols provide antioxidant properties, supporting cellular protection and moderate oxidative stress. Alkaloids suggest potential bioactive properties that could be explored for medicinal purposes, and the presence of proteins shows the nutritional value of Curcuma longa. The presence of Tannins, Terpenes, and Resins in the pure Curcuma longa underlines its potential as a therapeutic agent 34. However, the absence of Tannins, Terpenes, and Resins in the processed Curcuma longa is probably due to the refinement and purification processes employed.
The Cytotoxicity of Curcuma longa (Turmeric) pure and processed ethanolic extracts were assessed on NIH3T3 mouse fibroblast cell line using MTS assay. The results revealed significant variances in cell viability between the two extract types (Figure 3). Pure Curcuma longa exhibited high cell viability (88% and 98%). The processed Curcuma longa exhibited varying cytotoxicity patterns. At the concentration of 50mg/ml cell viability was significantly lower (35%) whereas, at 25mg/ml cell viability increased to 60%. These results highlight the importance of considering the source and composition of Curcuma longa when evaluating their safety and potential applications. Pure Curcuma longa appears safe for cells, while the processed Curcuma longa exhibits dose-dependent cytotoxicity. This study offers essential insights into the cytotoxicity patterns exhibited by Curcuma longa, extending our understanding of their safety and potential applications in Medicine and Biotechnology.
This study highlights Curcuma longa (Turmeric) antibacterial potential against respiratory pathogens, with varying MIC values emphasizing the need for standardized testing. Phytochemical analysis reveals its diverse composition, suggesting pharmacological significance. The presence of compounds like flavonoids suggests its therapeutic potential. The crude extract’s complex profile includes Tannins, Terpenes, and Resins. Furthermore, Cytotoxicity testing shows ethanolic extracts of pure Curcuma longa are safe for cells. Its antibacterial effects align with addressing antimicrobial resistance, contributing to universal health coverage with access to safe medicines.
The staff of the College of Medical Technology, Clinical Microbiology Lab, and Mr. Ziaullah.
The authors declare no conflict of interest.
HR Study design, plan of work, execution of the project, data analysis, and manuscript write-up; FI Study design, plan of work, project supervision, and manuscript critical review; MF data interpretation and manuscript review, QN Data compilation and manuscript review. AA Cytotoxic analysis and manuscript review FF Data analysis, data interpretation, and manuscript review.
- World Health Organization – Regional Office for the Eastern Mediterranean. Global Action Plan on Antimicrobial Resistance. Available from: http://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html.
- Institute for Health Metrics and Evaluation (IHME), University of Oxford. Global Bacterial Antimicrobial Resistance Burden Estimates 2019. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME); 2022. DOI: 10.6069/DBG1-V028.
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022; 399(10325): 629-655. DOI: 10.1016/S0140-6736(21)02724-0
- Torumkuney D, Jamil B, Nizamuddin S, van Hasselt J, Pirzada U, Manenzhe R. Country data on AMR in Pakistan in the context of community-acquired respiratory tract infections: links between antibiotic susceptibility, local and international antibiotic prescribing guidelines, access to medicine and clinical outcome. J Antimicrob Chemother. 2022; 77(Supplement_1):i18–25. DOI:10.1093/jac/dkac213
- Wang LM, Qiao XL, Ai L, Zhai JJ, Wang XX. Isolation of antimicrobial resistant bacteria in upper respiratory tract infections of patients. 3 Biotech. 2016;6(2):166. DOI :10.1007/s13205-016-0473-z
- Magesh H, Kumar A, Alam A, Sekar U. Identification of natural compounds which inhibit biofilm formation in clinical isolates of Klebsiella pneumoniae. 2013. Available from: https://nopr.niscpr.res.in/bitstream/123456789/21073/1/IJEB%2051 (9) %20764-772.pdf.
- Tangcharoensathien V, Chanvatik S, Sommanustweechai A. Complex determinants of inappropriate use of antibiotics. Bull World Health Organ. 2018; 96(2):141. DOI: 10.2471%2FBLT.17.199687
- Abiraami VS, Gowrie SU. Sprouts as functional food-an approach towards the identification of natural antibiotic resistance breakers. J Drug Deliv Ther. 2019; 9(1-s):23–35. DOI: 10.22270/jddt.v9i1-s.2240
- Yasunaka K, Abe F, Nagayama A, Okabe H, Lozada-Pérez L, López-Villafranco E, et al. Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J Ethnopharmacol. 2005; 97(2):293–299.DOI: 10.1016/j.jep.2004.11.014
- Satras KB. Prevalence and Susceptibility Analysis of Gram Negative Pathogens in Super Specialty Tertiary Care Centers, Pune in India from January 2018-January 2019. Microbiol Res J Int. 2019; 28(6):1–9. DOI: 10.9734/mrji/2019/v28i630150
- Aggarwal BB, Surh YJ, Shishodia S. The molecular targets and therapeutic uses of curcumin in health and disease. Vol. 595. Springer Science & Business Media; 2007.
- Ghiamati Yazdi F, Soleimanian-Zad S, Van Den Worm E, Folkerts G. Turmeric Extract: Potential Use as a Prebiotic and Anti-Inflammatory Compound? Plant Foods Hum Nutr. 2019; 74(3):293–299. DOI: 10.1007/s11130-019-00733-x
- Nwinee Z, Bako GD, Aliyu S, Yusuf JS, Korfii U. Antibacterial activity of herbal spices (Garlic and Turmeric) on Staphylococcus aureus and Escherichia coli. IOSR J Pharm Biol Sci 17 2. 2022; 39–43.
- Wayne PA. Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. 2010.
- Nagayama A, Yamaguchi K, Watanabe K, Tanaka M, Kobayashi I, Nagasawa Z. Final report from the Committee on Antimicrobial Susceptibility Testing, Japanese Society of Chemotherapy, on the agar dilution method. Journal of infection and chemotherapy. 2008; 14(5):383-392. DOI: 10.1007/s10156-008-0634-Z
- Alves TM, Silva AF, Brandão M, Grandi TS, Smânia ED, Smânia Júnior A, Zani CL. Biological screening of Brazilian medicinal plants. Memórias do Instituto Oswaldo Cruz. 2000; 95:367-373. DOI: 10.1590/S0074-02762000000300012
- ISO 20776-1. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices–part 1: broth microdilution reference methods for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases.
- EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infec. 2003; 9:1-7.
- National Committee for Clinical Laboratory Standards, Barry AL. Methods for determining bactericidal activity of antimicrobial agents: approved guideline. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999 Sep.
- Rasool R, Ganai BA, Akbar S, Kamili AN, Masood A. Phytochemical screening of Prunella vulgaris L.-an important medicinal plant of Kashmir. Pak J Pharm Sci. 2010 Oct 1; 23(4):399-402.
- Vaghasiya Y, Dave R, Chanda S. Phytochemical analysis of some medicinal plants from western region of India. Research Journal of Medicinal Plant. 2011; 5(5):567-576. DOI: 10.3923/rjmp.2011.567.576.
- Koleva II, Van Beek TA, Linssen JP, Groot AD, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002; 13(1):8-17. DOI: https://doi.org/10.1002/pca.611.
- Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC complementary and alternative medicine. 2010; 10(1):1-8. DOI: 10.1186/1472-6882-10-21
- Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: An overview. International Journal of Chemical Studies. 2020;8(2):603-608. DOI: 10.22271/chemi.2020.v8.i2i.8834.
- Kamiloglu S, Sari G, Ozdal T, Capanoglu E. Guidelines for cell viability assays. Food Frontiers. 2020; 1(3):332-349. DOI: 10.1002/fft2.44.
- Bergman P, Linde C, Pütsep K, Pohanka A, Normark S, Henriques-Normark B, Andersson J, Björkhem-Bergman L. Studies on the antibacterial effects of statins-in vitro and in vivo. PloS One. 2011;6(8):e24394. DOI: 10.1371/journal.pone.0024394.
- Zeng Z, Shen ZL, Zhai S, Xu JL, Liang H, Shen Q, Li QY. Transport of curcumin derivatives in Caco-2 cell monolayers. Eur J Pharm Biopharm. 2017;117:123-131. DOI: 10.1016/j.ejpb.2017.04.004.
- Li LM, Li J, Zhang XY. Antimicrobial and molecular interaction studies on derivatives of curcumin against Streptococcus pneumoniae which caused pneumonia. Electron J Biotechnol. 2016; 19:8-14. DOI: 10.1016/j.ejbt.2015.09.011.
- Karpiński TM, Adamczak A. Fucoxanthin—An antibacterial carotenoid. Antioxidants. 2019; 8(8):239. DOI: 10.3390/antiox8080239.
- Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340. DOI: 10.3390/molecules25061340.
- Nilani P, Duraisamy B, Dhamodaran P, Ravichandran S, Elango K. Effect of selected antiasthmatic plant constituents against microorganism causing upper respiratory tract infection. Anc Sci Life. 2010; 29(3):30-32.
- Adamczak A, Ożarowski M, Karpiński TM. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals. 2020; 13(7):153. DOI: 10.3390/ph13070153.
- Kesharwani P, Johnston TP, Sahebkar A. Anticancer potential of curcumin-cyclodextrin complexes and their pharmacokinetic properties. Int J Pharm. 2022:122474. DOI: 10.1016/j.ijpharm.2022.122474.
- Javed M, Shoaib M, Iqbal Z, Khan MA, Hussain S, Amjad M. Phytochemical and Biological Studies on Curcuma longa L. in Pattoki (Kasur), Pakistan: Chemical and Biological studies of Curcuma longa. PPASB. 2020; 57(2):59-66. Available from: https://ppaspk.org/index.php/PPAS-B/article/view/38
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
