By Reena Kumari Sunil1, Tariq Siddique1, Sunil Kumar1, Samina Shamim1
- Ziauddin University Hospital, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD13-2/009
How to cite: Sunil RK, Siddique T, Kumar S, Shamim S. Safety and Efficacy of Pre-Cytoreduction Prior to Definitive Multi-Agent Chemotherapy in Aggressive Lymphoma. Pak J Med Dent. 2024;13(2): 55-61. Doi: 10.36283/PJMD13-2/009
Background: Pre-cytoreduction refers to the administration of initial chemotherapy to reduce tumor burden prior to initiating definitive multi-agent chemotherapy. This study assessed the safety and efficacy of pre-cytoreduction prior to definitive multi-agent chemotherapy in aggressive lymphomas.
Methods: A prospective cohort study was done at Ziauddin Hospital, Karachi for six months. Patients of either gender, aged between 18-80 years diagnosed with aggressive lymphoma (Stage II-IV) were included. Patients having stage-I lymphoma, primary CNS lymphoma, HIV-linked Diffuse Large B Cell Lymphoma (DLBCL) transformed lymphoma, and other malignancies were excluded. The pre-cytoreduction group received a low dose of chemotherapeutic drug on the −6th day and prednisolone 100mg daily for 7 days (−6th day to day 0) followed by standard chemotherapy on day 1. Non-pre-cytoreduction group received chemotherapy without pre-cytoreduction. After chemotherapy’s first cycle, follow-up was done for 30 days. Anxiety assessment was done using the Hamilton Anxiety Rating Scale (HAM-A). For data analysis, SPSS v27 was used.
Results: From 120 patients, 27(22.5%) were diagnosed with Burkitt Lymphoma while 93(77.5%) with DLBCL. Pre-cytoreduction included prednisone, in 17(14.2%) patients, while 12(10%), 13(11%), and 10(8%) were given Vincristine, Prednisolone (VP), Cyclophosphamide, Prednisolone (CP) and Cyclophosphamide, Vincristine, and Prednisolone (CVP) respectively. There was a significant difference between both groups’ tumor lysis syndrome, bone marrow suppression, ECOG PS (Eastern Cooperative Oncology Group Performance Status), and other post-treatment complications (p<0.001).
Conclusion: Pre-cytoreduction was reported to be slightly better than conventional therapy in terms of compliance, with pre-cytoreduction improving patients’ performance status, decreased hospital emergency visits, febrile neutropenia risk, and improved treatment response rates.
Keywords: Drug Therapy, Lymphoma, Burkitt Lymphoma.
Diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma, two of the known aggressive lymphomas are highly malignant and fast-growing cancers that pose significant challenges to patients and healthcare professionals 1. The management of these lymphomas typically involves a combination of chemotherapy, targeted therapy, and radiation therapy. Starting chemotherapy in these patients with advanced stages of lymphoma poses them at risk of chemotherapy-related complications. Pre-cytoreduction has emerged as a promising strategy for optimizing the safety and efficacy of multi-agent chemotherapy in such cases of aggressive lymphomas 2.
Pre-cytoreduction refers to the administration of initial chemotherapy to reduce tumor burden before initiating definitive multi-agent chemotherapy 3. The rationale behind this approach is to minimize the risk of tumor lysis syndrome (TLS), improve performance status, and reduce the risk of febrile neutropenia, fatigue, diarrhea, fewer visits to the emergency department, and other side effects 4. Not only reducing these risks, cytoreduction also improves the functional status of patients in terms of both physical and mental health 5. The primary objective of pre-cyto reduction is to achieve a rapid and significant reduction in tumor burden, thereby reducing the risk of TLS and improving patient outcomes. This can be accomplished using various chemotherapeutic regimens, including high-dose corticosteroids, single-agent chemotherapy, or combination chemotherapy. The choice of pre-cytoreduction regimen depends on factors such as the type and stage of lymphoma, patient characteristics, and individual treatment goals 6.
Various studies have investigated the safety and efficacy of pre-cytoreduction in aggressive lymphomas, and the results have been encouraging 7. For instance, a study showed promising results in patients with Hodgkin Lymphoma and reported that using pre-cytoreduction therapy, an overall response rate of 74% and complete remission in 24% was observed 7. Another research reported a 5-year cancer-free survival rate in patients undergoing pre-cytoreduction for DLBCL at 87% 6. Another retrospective study conducted in 2019 evaluated the outcomes of DLBCL patients who received pre-cytoreduction with corticosteroids followed by multi-agent chemotherapy. The study found that pre-cyto reduction significantly reduced the incidence of TLS by around 20% and allowed for the safe administration of subsequent chemotherapy. Additionally, patients who underwent pre-cytoreduction had higher overall rates of response and improved progression-free survival in comparison to those who did not receive pre-cytoreduction 8.
In addition to reducing the risk of TLS, pre-cytoreduction has the potential to improve the efficacy of multi-agent chemotherapy by sensitizing tumor cells to subsequent treatment 9. Rapid tumor regression achieved through pre-cytoreduction may enhance the effectiveness of cytotoxic agents, leading to improved response rates and long-term outcomes. Furthermore, pre-cyto reduction allows for early assessment of tumor response, enabling clinicians to tailor subsequent treatment based on individual patients’ needs 10.
While pre-cytoreduction shows promise in optimizing the safety and efficacy of multi-agent chemotherapy, there are still several questions that need to be addressed such as optimal timing and duration of pre-cytoreduction, the choice of chemotherapeutic agents, and the impact of pre-cytoreduction on long-term survival outcomes 11,12. Future prospective studies and clinical trials are needed to further elucidate the role of pre-cytoreduction in the management of aggressive lymphomas. The use of pre-cytoreduction has been reported to be effective in other kinds of tumors as well, such as epithelial ovarian cancers, solid tumors, breast, colorectal, esophageal, and lung cancers. The rates of success vary based on the type of tumor, stage at diagnosis, and patient factors 4.
Pre-cytoreduction is an emerging strategy in treating aggressive lymphomas that aims to minimize the risk of TLS and chemotherapy-related complications (such as febrile neutropenia, diarrhea, fatigue, and visits to the emergency department) and improve the efficacy of subsequent multi-agent chemotherapy 13. Early evidence suggests that pre-cytoreduction is safe and effective, with potential benefits in terms of reduced TLS incidence and improved treatment response 14, 15. However, further research is warranted to optimize the use of pre-cytoreduction and determine its impact on long-term outcomes in patients with aggressive lymphomas. The objective of the research was to evaluate the effect of pre-cytoreduction prior to definitive multi-agent chemotherapy in aggressive lymphomas.
This prospective cohort study was carried out at the Ziauddin Medical University Hospital, Karachi by recruiting 60 patients using OpenEpi software and a non-probability purposive sampling technique for six months from February to July 2023, after approval by the Ethical Review Committee of Ziauddin Medical University Hospital, Karachi. (Reference Code: 6420123RKONC). Patients were in two groups; Group A included those patients that had undergone pre-cytoreduction while in Group B; patients without pre-cytoreduction (standard treatment) were included.
Patients of either gender aged between 18-80 years of age diagnosed with aggressive lymphoma (Stage II-IV) were included and enlisted through the ward and ICU of the hospital. Patients below the age of 18 years having stage I lymphoma, primary CNS lymphoma, HIV-associated DLBCL, and with a previous history of other malignancies such as low-grade lymphoma with transformation to DLBCL was excluded from the study. In addition, patients with known allergies were also excluded. Informed consent was sought from all patients who were included in the study. The drugs used for pre-cytoreduction therapy included Vincristine, Prednisolone (VP), Cyclophosphamide, Prednisolone (CP), and Cyclophosphamide, Vincristine, and Prednisolone (CVP) respectively.
After ethical approval from the Ethical Review Committee, patients were divided into 2 groups. Group A Pre-cytoreduction received a low dose of chemotherapeutic drug on the −6th day and prednisolone 100 mg daily for 7 days (−6th day to day 0). Pre-cytoreduction was followed by CHOP/R-CHOP (Rituximab) chemotherapy on day 1. Non-pre-cytoreduction group (Group B) received chemotherapy without pre-cytoreduction. Both groups were followed up for 30 days post-first cycle chemotherapy. Assessment of anxiety was done using the Hamilton Anxiety Rating Scale (HAM-A) 16. Assessment of anxiety was done only for evaluating the patient’s anxiety status to be at par with the non-pre-cytoreduction group and since it was not the research objective, therefore its findings were not part of the results and discussion. In both groups, patients’ demographics, baseline laboratory, and/or radiological investigations were recorded before and after treatment. After treatment, patients were followed up and their overall health status was reported along with any adverse events occurring post-treatment.
SPSS version 27 was used for statistical analyses. For qualitative variables, frequency and percentages were reported while for quantitative variables, mean and standard deviation was recorded. Chi-square and student t-tests were used for the comparison of the various clinical variables as appropriate. p-value of <0.05 was considered significant.
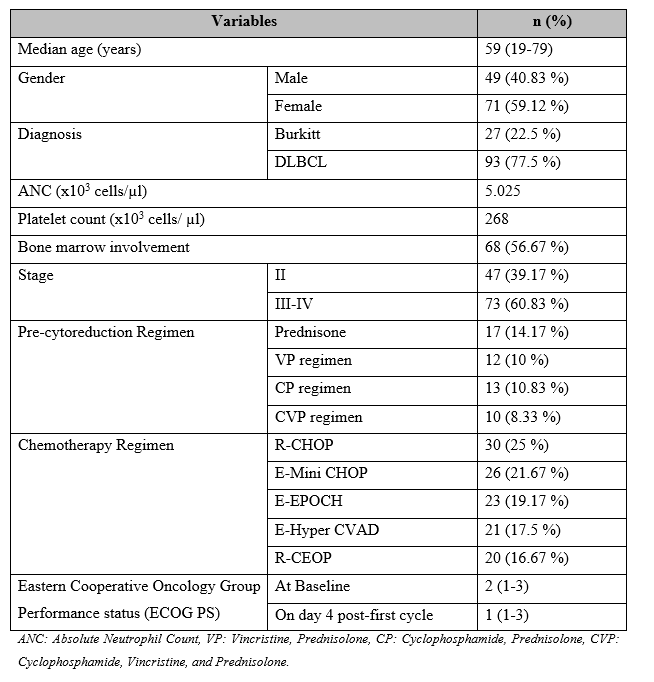
A total of 120 patients were included, of which 52 were included in the pre-cytoreduction group and 68 in the non-pre-cytoreduction group. The median age of patients was 59 (19-79) years with 49 (40.8%) males and 71 (59.1%) females. A total of 27 (22.5 %) patients were diagnosed with Burkitt lymphoma while 93 (77.5%) were with DLBCL. The median value of Absolute Neutrophil Count and platelet count was 5.025 x103 cells/ µl and 268 x103 cells/ µl respectively. A total of 47 (39.2%) patients were in stage II of cancer while 73 (60.83%) were in stage III-IV. Moreover, 68 (56.7%) patients were reported to have bone marrow involvement.
With regards to the pre-cytoreduction regimen, 17 (14.2%) patients were administered prednisone only, 12 (10%) patients were given the VP regimen, 13 (10.8%) were given the CP regimen, and 10 (8.3%) were administered the CVP regimen. In terms of the chemotherapy regimen, R-CHOP was administered in 30 (25%) patients, E-Mini CHOP was given in 26 (21.7%) patients, E-EPOCH 23 (19.2%) patients, E-Hyper CVAD in 21 (17.5%) patients, and R-CEOP in 20 (16.7%) patients. Median Eastern Cooperative Oncology Group Performance status (ECOG PS) at baseline was 2 (1-3) and on the 4th day, post-first cycle was 1 (1-3) (Table 1).
Table 1: Baseline demographics of patients included in the study (n=120)
Regarding cross-tabulation of various demographics in the pre-cytoreduction vs non-pre-cytoreduction group, a significant difference between the two groups was reported in terms of diagnosis (p=0.05), bone marrow involvement (p=0.04) and Eastern Cooperative Oncology Group Performance status (ECOG PS) (p=0.05) (Table 2).
Table 2: Cross-tabulation of various demographics in pre-cytoreduction vs non-pre-cytoreduction group (n=120)

Post-pre-cytoreduction and post-chemotherapy, neutropenia was observed in 14 (27%) of patients in the pre-cytoreduction group while in 29 (4%) of patients in the non-pre-cytoreduction group. Thrombocytopenia was reported in 15 (29 %) of patients in the pre-cytoreduction group while in 32 (47%) of patients in the non-pre-cytoreduction group. In the pre-cytoreduction group, anemia was recorded in 17 (33%) patients while in 28 (41%) patients in the non-pre-cytoreduction group. Early recovery was observed in 33 (64%) of the patients in pre- cytoreduction group and 33 (49%) patients in the non-pre-cytoreduction group. Tumor lysis syndrome was observed in 11 (22%) patients in pre- cytoreduction group and 20 (29%) patients in the non-pre-cytoreduction group. Re-admission within 30 days was observed in 06 (12%) patients in pre- cytoreduction group while in 14 (21%) patients in the non-pre-cytoreduction group. A 30-day mortality was reported in 03 (6%) patients in pre- cytoreduction group and in 07 (11%) patients in the cytoreduction – group (Figure 1).

Figure 1: Graphical representation of complications reported in pre-cytoreduction vs non-pre-cytoreduction group through independent t-test (p<0.001)
With regards to patient scores on the Hamilton Anxiety scale, a mild score (<17) was observed in 09 (18%) of patients in the pre-cytoreduction group and 14 (21%) of patients in the non-pre-cytoreduction group. A total of 14 (27%) of patients in the pre-cytoreduction group and 21 (31%) in the non-pre-cytoreduction group reported mild to moderate scores (18-24) while severe (>25) scores were reported in 04 (7%) of patients in pre-cytoreduction group and in 11 (16%) in the non-pre-cytoreduction group (Figure 2).

Figure 2: Graphical representation of patient scores on the Hamilton Anxiety scale in pre-cytoreduction vs non-pre-cytoreduction group through chi-square test (p<0.001)
Aggressive lymphomas, such as diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma, are challenging malignancies that require intensive treatment strategies 17. The use of pre-cytoreduction, an approach involving initial chemotherapy to reduce tumor burden before definitive multi-agent chemotherapy, has emerged as a promising strategy to improve the safety and efficacy of treatment 18.
One of the primary concerns in the management of aggressive lymphomas is the risk of tumor lysis syndrome (TLS). The rapid destruction of cancer cells during chemotherapy can lead to the release of intracellular contents, resulting in electrolyte imbalances and organ dysfunction. Pre-cytoreduction aims to mitigate the risk of TLS by reducing the tumor burden before initiating multi-agent chemotherapy and to improve the performance status of patients. 19. In line with our study, it is also mentioned that pre-cytoreduction decreases hospital emergency department visits and risk of febrile neutropenia.
A study reported a lower frequency of death due to lymphoma progression in the pre-cytoreduction group (42%) as compared with the non-pre-cytoreduction group (75%). Likewise, a higher frequency of infections and toxicity were reported in the non-pre-cytoreduction group (22%) in comparison to only 04 % in the pre-cytoreduction group 8. Multiple studies have demonstrated the safety of pre-cytoreduction in aggressive lymphomas20. These findings suggest that pre-cytoreduction can effectively reduce TLS-associated complications and improve patient safety during treatment 21.
In addition to its safety profile, pre-cytoreduction has shown promise in enhancing the efficacy of subsequent multi-agent chemotherapy. By reducing tumor burden before initiating definitive treatment, pre-cytoreduction allows for better tumor response rates and improved long-term outcomes 22. Studies have reported favorable outcomes with pre-cyto reduction in aggressive lymphomas. A retrospective analysis by Jones et al. in 2020 evaluated DLBCL patients treated with pre-cytoreduction followed by multi-agent chemotherapy. The study demonstrated higher overall response rates (by around 40-50%) and improved progression-free survival (by 20% on average) in patients who received pre-cytoreduction compared to those who did not receive it 23. These findings suggest that pre-cytoreduction can sensitize tumor cells to subsequent treatment, leading to enhanced treatment responses 24,25.
Prospective studies and clinical trials are needed to address these gaps and provide evidence-based guidelines for the use of pre-cytoreduction in aggressive lymphomas. These studies should focus on long-term survival outcomes, as well as the impact of pre-cytoreduction on quality of life and treatment-related toxicities. Furthermore, the identification of predictive biomarkers to identify patients who would benefit the most from pre-cytoreduction would be valuable in tailoring treatment strategies. Nevertheless, further research is required to optimize the use of pre-cytoreduction, including determining the optimal timing, duration, and choice of chemotherapeutic agents.
Pre-cyto reduction is a promising strategy in the management of aggressive lymphoma which this study demonstrated. According to the results of the study, pre-cytoreduction was reported to be better than conventional therapy in terms of compliance, with pre-cytoreduction improving the performance status of patients, decreasing hospital emergency department visits, risk of febrile neutropenia, other chemotherapy-related complications and improved treatment response rates. The existing evidence suggests that pre-cyto reduction is safe and effective, with potential benefits in terms of reduced TLS incidence, in addition, pre-cytoreduction improves the performance status of patients and also decreases hospital emergency department visits and risk of febrile neutropenia and other chemotherapy-related complications and improve treatment response rates.
None
There is no conflict of interest among authors.
The study approval was taken from the Ethical Review Committee of Ziauddin Medical University Hospital, Karachi. (Reference Code: 6420123RKONC).
RKS: Concept, design of the study, and reviewing the manuscript critically, TS: Drafting, SU: Data analysis, SS: Final approval of version.
- Mugnaini EN, Ghosh N. Lymphoma. Primary Care: Clinics in Office Practice. 2016;43(4):661-675. https://doi.org/10.1016/j.pop.2016.07.012
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-hodgkin lymphoma. The lancet. 2017 ;390(10091):298-310. https://doi.org/10.1016/S0140-6736(16)32407-2
- Aslam W, Habib M, Aziz S. Clinicopathological Spectrum of Hodgkin’s and Non-Hodgkin’s Lymphoma: A Tertiary Care Cancer Hospital Study in Pakistan. Cureus. 2022 ;14(6) :1-6. DOI: 10.7759/cureus.25620
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA: a cancer journal for clinicians. 2018;68(2):116-132. https://doi.org/10.3322/caac.21438
- Pervez S. Non-hodgkin lymphoma (NHL) in Pakistan. International Journal of Molecular and Cellular Medicine 2012;1(1):62. http://ijmcmed.org/article-1-29-en.html
- Lakshmaiah KC, Asati V, Babu K G, Jacob LA, MC SB, KN L, et al. Role of prephase treatment prior to definitive chemotherapy in patients with diffuse large B‐cell lymphoma. European Journal of Haematology. 2018;100(6):644-648. https://doi.org/10.1111/ejh.13068
- Malpica L, Mufuka B, Galeotti J, Tan X, Grover N, Clark SM, et al. A retrospective study on prephase therapy prior to definitive multiagent chemotherapy in aggressive lymphomas. Leukemia & lymphoma. 2020;61(6):1508-1511. https://doi.org/10.1080/10428194.2020.1725505
- Cencini E, Tucci A, Puccini B, Cavallo F, Luminari S, Usai SV, et al. The elderly prognostic index predicts early mortality in older patients with diffuse large B‐cell lymphoma. An ad hoc analysis of the elderly project by the Fondazione Italiana Linfomi. Hematological Oncology. 2023;41(1):78-87. https://doi.org/10.1002/hon.3081
- Schmittlutz K, Marks R. Current treatment options for aggressive non-Hodgkin lymphoma in elderly and frail patients: practical considerations for the hematologist. Therapeutic Advances in Hematology. 2021; 12:2040620721996484. https://doi.org/10.1177/2040620721996484
- Wei Y, Yang Q, Qin Q, Chen Y, Quan X, Wei J, et al. Profiling of the Risk Factors and Designing of a Model to Identify Ischemic Stroke in Patients with Non-Hodgkin Lymphoma: A Multicenter Retrospective Study. European Neurology. 2020;83(1):41-48. https://doi.org/10.1159/000506046
- Di M, Huntington SF, Olszewski AJ. Challenges and opportunities in the management of diffuse large B‐cell lymphoma in older patients. The Oncologist. 2021 ;26(2):120-132. https://doi.org/10.1002/onco.13610
- Gobba SM, Moccia AA, Conconi A, Diem S, Cascione L, von Hohenstaufen KA, et al. Survival and prognostic factors in very elderly patients (pts) with diffuse large B-cell lymphoma (DLBCL): a retrospective analysis of 281 patients over 80 years. Annals of Oncology. 2015; 26:135. https://doi.org/10.1093/annonc/mdv348.11
- Eertink JJ, Heymans MW, Zwezerijnen GJ, Zijlstra JM, de Vet HC, Boellaard R. External validation: a simulation study to compare cross-validation versus holdout or external testing to assess the performance of clinical prsediction models using PET data from DLBCL patients. EJNMMI research. 2022;12(1):1-8. https://doi.org/10.1186/s13550-022-00931-w
- Di M, Huntington SF, Olszewski AJ. Challenges and opportunities in the management of diffuse large B‐cell lymphoma in older patients. The Oncologist. 2021;26(2):120-132. https://doi.org/10.1002/onco.13610
- Kesavan M, Eyre TA, Collins GP. Front-line treatment of high-grade B cell non-Hodgkin lymphoma. Current Hematologic Malignancy Reports. 2019; 14:207-218. https://doi.org/10.1007/s11899-019-00518-8
- Carrozzino D, Patierno C, Fava GA, Guidi J. The Hamilton rating scales for depression: a critical review of clinimetric properties of different versions. Psychotherapy and psychosomatics. 2020;89(3):133-150. https://doi.org/10.1159/000506879.
- King RL, Hsi ED, Chan WC, Piris MA, Cook JR, Scott DW, Swerdlow SH. Diagnostic approaches and future directions in Burkitt lymphoma and high-grade B-cell lymphoma. Virchows Archiv. 2023;482(1):193-205. https://doi.org/10.1007/s00428-022-03404-6
- Kusamura S, Torres Mesa PA, Cabras A, Baratti D, Deraco M. The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Annals of surgical oncology. 2016; 23:1468-1473. https://doi.org/10.1245/s10434-015-4962-9
- Gangireddy M, Shrimanker I, Nookala VK, Peroutka KA. Spontaneous tumor lysis syndrome in diffuse large B-cell lymphoma: early diagnosis and management. Cureus. 2019;11(5). DOI: 10.7759/cureus.4679
- Smith SD, Till BG, Shadman MS, Lynch RC, Cowan AJ, Wu QV, et al. A. Pembrolizumab with R‐CHOP in previously untreated diffuse large B‐cell lymphoma: potential for biomarker-driven therapy. British journal of hematology. 2020;189(6):1119-1126. https://doi.org/10.1111/bjh.16494
- Turaga KK, Gamblin TC, Alexander HR, Edwards R, Bartlett DL. Regional Therapies for Advanced Cancer: Update for 2016. Annals of Surgical Oncology. 2016; 23:1452-1453. https://doi.org/10.1245/s10434-015-5068-0
- Kaiser U, Uebelacker I, Abel U, Birkmann J, Trumper L, Schmalenberg H, et al. Randomized study to evaluate the use of high-dose therapy as part of primary treatment for” aggressive” lymphoma. Journal of clinical oncology. 2002 ;20(22):4413-4419. DOI: 10.1200/JCO.2002.07.075
- Jones SJ, Brooks-Wilson A. Anticipation in multiple-case lymphoid cancer families after controlling for ascertainment biases. Leukemia & Lymphoma. 2021 ;62(13):3147-3151. https://doi.org/10.1080/10428194.2021.1948026
- Olszewski AJ, Ollila T, Reagan JL. Time to treatment is an independent prognostic factor in aggressive non‐Hodgkin lymphomas. British journal of hematology. 2018;181(4):495-504. https://doi.org/10.1111/bjh.15224
- Zayac AS, Olszewski AJ. Burkitt lymphoma: bridging the gap between advances in molecular biology and therapy. Leukemia & lymphoma. 2020;61(8):1784-1796. https://doi.org/10.1080/10428194.2020.1747068
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
