By Nauman Ismat Butt1, Muhammad Sohail Ajmal Ghoauri2
- Medicine & Allied Department, Azra Naheed Medical College, Superior University, Lahore, Pakistan
- Medicine & Neurology Department, Bahawal Victoria Hospital, Quaid-e-Azam Medical College Bahawalpur, Pakistan
DOI: https://doi.org/10.36283/PJMD13-2/017
How to cite: Butt NI, Ghoauri MSA. Co-existence of Systemic Lupus Erythematosus and Celiac Disease: A Case Report. Pak J Med Dent.2024;13(2): 112-115. Doi: 10.36283/PJMD13-2/017
Systemic Lupus Erythematosus (SLE) is an autoimmune multisystem disease usually affecting mucocutaneous, hematologic, and renal organs. Whereas, Celiac Disease is a chronic digestive and immune disorder of the small intestine leading to diarrhea and malnutrition. We report the case of an 18-year-old girl with chronic diarrhea for 1 year with bilateral feet swelling, photosensitive skin rash over sun-exposed areas, recurrent oral ulcers, marked hair fall, generalized lethargy, and fatigue. On examination, she was underweight having malar butterfly rash, oral ulcers, alopecia, conjunctival pallor, and bilateral pitting pedal edema. Investigations revealed raised ESR, microcytic anemia due to iron deficiency, and low serum albumin. Duodenal biopsy showed villous atrophy, lamina propria inflammation, and intraepithelial lymphocytosis. The autoimmune profile revealed positive anti-tissue transglutaminase (tTG-IgA and tTG-IgG), ANA, anti-DsDNA, and Anti-Sm antibodies with low serum C3 and serum C4 levels. A diagnosis of co-existing Celiac Disease and SLE was made and was managed with a gluten-free diet, steroids, and hydroxychloroquine.
Keywords: Celiac Disease, Endoscopy, Prednisolone, Systemic Lupus Erythematosus.
Being an autoimmune multi-system disease, the clinical presentation of Systemic Lupus Erythematosus (SLE) varies from mild mucocutaneous features to potentially life-threatening multi-organ disease involving renal, musculoskeletal, and cardiovascular organs1,2. Between the 1950s and the 2000s, the overall SLE survival significantly improved from 74.8% to 94.8% for the overall 5-year survival and 63.2% to 91.4% for the overall 10-year survival.1 However, the survival improvement slowed down between 1980 and 1990. Neuropsychiatric and renal involvement in SLE patients negatively impacted the overall 5-year survival while neuropsychiatric involvement remained so for the 10-year survival for the past 50 years1. Additionally, the frequency of neuropsychiatric involvement has been increasing significantly over the past 5 decades1. Due to advances in diagnosis facilities and management options, mortality and morbidity caused by SLE have been reduced. However, delay in diagnosis and inadequate disease control still results in significant disease burden, poor quality of life, and mortality.
On the other hand, Celiac disease is a chronic digestive and immune disorder of the small intestine leading to recurrent or chronic diarrhea, nausea, vomiting, and indigestion which usually results in malnutrition3. Celiac disease has been linked to various autoimmune diseases including type-I diabetes mellitus, Addison’s disease, thyroid disorders, autoimmune hepatitis, and Sjogren’s syndrome, however, co-existence of celiac disease with SLE is rare4. The association of celiac disease and SLE has been suggested in a few recent studies but the mechanism of their co-existence remains poorly understood5,6. Soltani et al. evaluated 130 SLE patients to document celiac disease in 3%, which is 5 times higher as compared to celiac disease prevalence in the general population4.
Herein we present the case of a young girl who presented with chronic diarrhea, photosensitivity, oral ulcers, alopecia, and iron deficiency anemia, subsequently revealed to have co-existent celiac disease and SLE.
An 18-year-old girl presented to CMA Hospital, Azra Naheed Medical College, Lahore, with chronic diarrhea for the last 1 year with the frequency of 4-6 large volume stools per day. There was no history of nausea, vomiting, abdominal pain, and passing mucus, worms, or blood in stools. On exploration of history, she reported bilateral feet swelling, photosensitive skin rash over sun-exposed areas, recurrent oral ulcers, marked hair fall, generalized lethargy, and fatigue. No history of fever, genital ulcers, renal stones, puffy hands, Raynaud’s phenomena, urinary complaints, or neuropsychiatric features were reported. She was unmarried and did not smoke or use illicit drugs. There was no family history of gastrointestinal or rheumatic diseases. On examination, the patient was underweight (35 kgs, BMI 14.1 kg/m2) with malar butterfly rash, 3 round oral ulcers over buccal mucosa, marked alopecia, and conjunctival pallor. Bilateral pitting edema was present extending up to mid-shins. There was no cyanosis, clubbing, lymphadenopathy, or parotid swelling. The abdomen was soft, and non-tender, with no palpable viscera and negative signs of free fluid in the peritoneal cavity. Examinations of the precordium, chest, and nervous system were unremarkable.
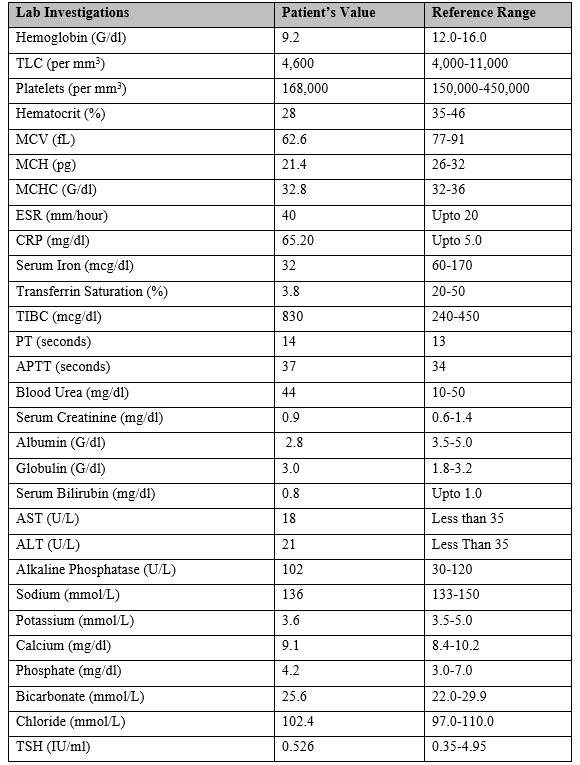
As shown in Table 1, on investigation CBC revealed microcytic anemia with hemoglobin 9.2 g/dl and normal TLC and platelet counts. Erythrocyte Sedimentation Rate (ESR) was raised at 40 mm/hour with normal C-reactive protein (CRP). Serum albumin was low (2.8 g/dl) but Liver Function Tests (LFTs) were normal. Renal Function Tests (RFTs), serum electrolytes, serum Thyroid Stimulating Hormone (TSH), and urinalysis were also normal. Serum iron (32 mcg/dl) and transferrin saturation (3.8%) were low with high serum Total Iron Binding Capacity (TIBC: 830 mcg/dl) indicating iron deficiency. Serologies for Syphilis, Human Immunodeficiency Virus (HIV), and Hepatitis B and C were negative. An upper gastrointestinal endoscopy was done for duodenal biopsy which showed villous atrophy, lamina propria inflammation, and intraepithelial lymphocytosis on histopathology. Trans-thoracic echocardiography and ultrasound abdomen and pelvis were within normal parameters. An autoimmune profile was ordered which revealed positive anti-tissue transglutaminase (tTG-IgA and tTG-IgG), ANA, anti-DsDNA, and Anti-Sm antibodies with low serum complements (C3 and C4) levels but negative ENA profile.
Table 1: Lab investigations of the patient
Based on clinical and laboratory findings, a diagnosis of co-existing Celiac Disease and SLE was made. Counseling and nutritional guidance regarding gluten-free diet were done. She was started on pulse intravenous methylprednisolone 500mg/day for 5 days followed by enteric-coated oral prednisolone (10mg/day) with gradual dose tapering over 6 weeks. In addition, she was prescribed hydroxychloroquine (5mg/kg body weight per day once diarrhea settled), sunblock SPF 60, iron, calcium, and vitamin D supplements. On follow-up at 12 weeks, the patient was asymptomatic and reported a weight gain of 10 kgs (BMI 18.2 kg/m2). She was tolerating hydroxychloroquine without any adverse effects and following a gluten-free diet with good compliance. By 12-week follow-up, her hemoglobin level, ESR, and serum albumin were within normal limits.
Celiac disease is usually diagnosed by serum anti-tissue transglutaminase (tTG-IgA and tTG-IgG) antibodies and small intestine biopsy showing characteristic histological findings of villous atrophy7. Our patient had positive anti-tissue transglutaminase antibodies in addition to villous atrophy on intestinal biopsy leading to a diagnosis of Celiac disease. Having a specificity and sensitivity of 92% and 97% respectively, the Systemic Lupus International Collaborating Clinics (SLICC) criteria is usually employed to diagnose SLE8. SLE is diagnosed in the presence of >4 criteria, at least 1 immunological criterion, and 1 clinical criterion8. Based on the SLICC criteria, our patient had a score of 6: oral ulcers, alopecia, photosensitivity, positive ANA, positive Anti-DsDNA antibodies, and low serum complement (C3 and C4). In addition, our patient also had iron deficiency anemia which is associated with both SLE (chronic inflammation) and celiac disease (malnutrition and malabsorption).
There are no specific guidelines about the treatment of co-existence of SLE with celiac disease and both diseases are treated according to the patient’s signs and symptoms. The mainstay of treatment of Celiac disease relies on a gluten-free diet9. Gluten is a protein found in wheat, rye, barley, and triticale. Avoidance of gluten-containing food usually leads to clinical improvement in patients with Celiac disease. Whereas, treatment of SLE depends on disease severity and the organ systems involved. The first-line agents used are corticosteroids due to rapid onset of action and powerful anti-inflammatory actions10. Hydroxychloroquine is used to treat fatigue, and musculoskeletal and mucocutaneous manifestations of SLE and also improves long-term survival by protecting against thrombosis, irreversible organ damage, and bone mass loss, and aids in preventing disease flare11,12. Other treatment options include conventional DMARDs (azathioprine, methotrexate, mycophenolate) and biologic DMARDs (rituximab, belimumab) depending on disease severity and organ-system involvement13,14. Our patient was started on a gluten-free diet, steroids, and hydroxychloroquine which resulted in clinical improvement and weight gain.
In conclusion, high clinical evaluation is required to diagnose the co-existence of two autoimmune disorders as proper recovery may not be achieved until both diseases are controlled. Though these two diseases are seldom associated with each other, the likelihood that a patient may be afflicted with both diseases at the same time does exist. Additional researches are required in order to determine the true prevalence of this coexistence.
None
No conflict of interest exists.
Detailed informed consent was taken from the patient and her parents before data collection and manuscript writing. There are no ethical issues in the present case report.
NIB was involved in the conception of study, patient assessment, data collection, literature review, manuscript writing. MSAG was involved in the conception of the study, literature review, critical revisions, and proofreading.
- Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 2012;41(6):830-839. doi: 10.1016/j.semarthrit.2011.11.002
- Butt NI, Ashfaq F, Mahmood K, Aftab S, Anwar A, Saeed M. Lupus Vasculitis. J Ayub Med Coll Abbottabad. 2023;35(3):466–469. doi.org/10.55519/JAMC-03-10849
- Lupu VV, Jechel E, Mihai CM, Mitrofan EC, Lupu A, Starcea IM, et al. Connection between Celiac Disease and Systemic Lupus Erythematosus in Children-A Development Model of Autoimmune Diseases Starting from What We Inherit to What We Eat. Nutrients. 2023;15(11):2535. doi: 10.3390/nu15112535
- Soltani Z, Baghdadi A, Nejadhosseinian M, Faezi ST, Shahbazkhani B, Mousavi SA, et al. Celiac disease in patients with systemic lupus erythematosus. Reumatologia. 2021;59(2):85-89. doi: 10.5114/reum.2021.105416
- Li T, Feng Y, Wang C, Shi T, Huang X, Abuduhadeer M, et al. Causal relationships between autoimmune diseases and celiac disease: A Mendelian randomization analysis. Biotechnol Genet Eng Rev. 2023:1-16. doi: 10.1080/02648725.2023.2215039. Epub ahead of print.
- Wang Q, Jia S, Lu Q, Zhao M. Systemic lupus erythematosus is associated with the risk of coeliac disease: a Mendelian randomisation study. Autoimmunity. 2023;56(1):2250103. doi: 10.1080/08916934.2023.2250103
- Ma Y, Zhuang D, Qiao Z. Dual threat of comorbidity of celiac disease and systemic lupus erythematosus. J Int Med Res. 2021;49(5):3000605211012258. doi: 10.1177/03000605211012258
- Aringer M. EULAR/ACR classification criteria for SLE. Semin Arthritis Rheum. 2019;49(3S):S14-S17. doi: 10.1016/j.semarthrit.2019.09.009
- Kelly CP, Bai JC, Liu E, Leffler DA. Advances in diagnosis and management of celiac disease. Gastroenterology. 2015;148(6):1175-1186. doi: 10.1053/j.gastro.2015.01.044
- Chaichian Y, Weisman MH, Simard JF. Pulse dose steroid experience among hospitalized patients with systemic lupus erythematosus: a single-center feasibility study. Clin Rheumatol. 2021;40(4):1317-1320. doi: 10.1007/s10067-021-05644-4
- Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155-166. doi: 10.1038/s41584-020-0372-x
- Butt NI. Subacute Cutaneous Lupus Erythematosus in a Patient with Mixed Connective Tissue Disease. JCPSP Case Rep 2023; 1: 9-11. doi: 10.29271/jcpspcr.2023.9
- Golder V, Tsang-A-Sjoe MWP. Treatment targets in SLE: remission and low disease activity state. Rheumatology (Oxford). 2020;59(Suppl5):v19-v28. doi: 10.1093/rheumatology/keaa420
- Tunnicliffe DJ, Singh-Grewal D, Kim S, Craig JC, Tong A. Diagnosis, Monitoring, and Treatment of Systemic Lupus Erythematosus: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res (Hoboken). 2015;67(10):1440-1452. doi: 10.1002/acr.22591
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
