By Soha Haque1, Ambrina Khatoon2, Kauser Ismail1, Zahida Memon1, Faisal Iqbal Afridi3, Zara Aslam4, Raza Shah4
- Pharmacology Department, Ziauddin University, Karachi, Pakistan
- Molecular Medicine Department, Ziauddin University, Karachi, Pakistan
- Microbiology Department, Dr Ziauddin Hospital, Karachi, Pakistan
- E.J Research Institute of Chemistry, International Centre for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD13-2/005
How to cite: Haque S, Khatoon A, Ismail K, Memon Z, Afridi F I, Aslam Z, Shah R. Carvacrol Silver Nanoparticles – An Effective Antibacterial Against Carbapenem-Resistant Acinetobacter Isolates Unaided and With Meropenem. Pak J Med Dent. 2024;13(2): 22-30. Doi: 10.36283/PJMD13-2/005
Background: Acinetobacter species are a serious clinical challenge owing to their established resistance to carbapenems. This study aimed to explore Carvacrol silver nanoparticles’ activity against carbapenem-resistant Acinetobacter and their interaction with meropenem to develop effectual treatment.
Methods: An in-vitro experimental study was conducted from February 2021 to January 2022. The minimum inhibitory concentration (MIC) of Carvacrol silver nanoparticles was checked using the broth macrodilution method. The results were further corroborated by performing the agar well diffusion method on 50 isolates of carbapenem-resistant Acinetobacter to observe the zone of inhibition (ZOI). The interaction of Carvacrol silver nanoparticles with meropenem (synergistic/additive/antagonistic) was assessed by checkerboard assay. SPSS vr24 was used. Kruskal-Wallis ANOVA with pair-wise comparison analysis was applied to compare different groups. p-value<0.05 was considered significant.
Results: The study showed that older males were mostly affected and the majority of the isolates were from the tracheal secretions and collected from the Medical ICU. MIC of Carvacrol silver nanoparticles was found to be 0.04-0.16mg/ml. On agar well diffusion, the ZOI of Carvacrol silver nanoparticles was in the range of 0-20mm compared to meropenem (0) and colistin (0-14mm) with a p-value of 0.00. Checkerboard assay revealed additive interaction between Carvacrol silver nanoparticles and meropenem as the Fractional inhibitory concentration was calculated to be 1.25.
Conclusion: Carvacrol silver nanoparticles have shown the potential ability to combat carbapenem-resistant Acinetobacter and may prove as an effective antibacterial agent in the future. Furthermore, the additive interaction of Carvacrol silver nanoparticles with meropenem points toward the possible revival of carbapenems against Acinetobacter
Keywords: Carvacrol, Acinetobacter, Carbapenems, Anti-Bacterial Agents.
The gram-negative genus Acinetobacter is currently considered a troublesome group of organisms by clinicians worldwide. They are the major cause of serious hospital-acquired infections such as ventilator-associated pneumonia (VAP), septicemia, endocarditis, meningitis, urinary tract & wound infections which are difficult to treat and subsequently associated with increased mortalities. These organisms are unusual for their exceptional antibiotic resistance mechanism as they withstand the action of nearly all available antibiotics1.
The emergence of multi-drug resistant (MDR), extensive drug-resistant (XDR), and even pan-drug resistant (PDR) isolates of Acinetobacter across the globe has threatened the health care professional as there are severely limited therapeutic options and it has forced clinicians to switch to antibiotics with deleterious effects such as Polymyxins 2. World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have also accepted the menace of antibiotic resistance in bacteria endorsing the need for newer compounds capable of combating these resistant bugs3.
Carvacrol is a monoterpenic phenol found in essential oils of plants belonging to the Lamiaceae family such as Origanum, Thymus & Satureja. It is regarded as safe for human consumption and is being used as an additive in food formulations 4. In literature, Carvacrol is reported to possess useful biological activities such as antibacterial, antifungal & anticancer. Studies have shown its activity against Salmonella, Pseudomonas, Klebsiella, and others 5,6. Green synthesis of silver nanoparticles using different plant extracts and biomolecules has attracted much attention from researchers to resolve the plight of antimicrobial resistance with less toxicity 7. Silver nanoparticles (AgNPs) have a large surface area to volume ratio, containing around 15-20,000 silver atoms and their dimensions are smaller than 100nm which enables them to effectively kill bacterial cells at lower concentrations 8. In various studies, AgNPs have proved to be effective against E. coli, Staphylococcus, Pseudomonas, Salmonella, and others 9,10. Nowadays many commercially available products in the market contain nanosilver such as Acticoat (nanosilver-coated antibacterial dressing), Silver line (ventricular catheter instilled with nanosilver), and Silvasorb (antibacterial gel for wounds) 11.
Presently, there is no general agreement regarding the toxicity of AgNPs, with literature reporting varying results depending on the dose, period of exposure, stability, and age of the particles 12. Consequently, this study was designed to explore the antibacterial activity of AgNPs synthesized using conjugation with Carvacrol i.e., Carvacrol silver nanoparticles (CrAgNPs) against the Carbapenem-resistant Acinetobacter (CRA) species, and also to assess the combined activity of nanoparticles and meropenem to ascertain the possible revival of the latter against it.
This was an in vitro experimental study conducted from February 2021 to January 2022 on 50 isolates of CRA isolates. The sample size was calculated using a Continuous outcome noninferiority sealed envelope calculator with a 5% significance level and 90% power of the test. Carvacrol was purchased from Ambeed, U.S.A. Preformed CrAgNPs were provided by Hussain Ebrahim Jamal Research Institute of Chemistry (HEJRIC), International Center for Chemical and Biological Sciences (ICCBS), Karachi University. Mueller Hinton broth (MHB) & Mueller Hinton Agar (MHA) were purchased from Oxoid, U.K. CRA isolates were collected from the Microbiology lab of Dr. Ziauddin Hospital, North Campus, Karachi. Demographic data such as gender, age, source of infection, and ward association of the clinical isolates were also noted at the time of collection.
All the presumptive culture plates of Acinetobacter were further verified by Gram’s staining and different biochemical tests. On Gram’s staining these appear as gram –ve coccobacilli and they form colorless, mucoid, non-glucose fermenting colonies on McConkey’s agar while on biochemical tests they present as oxidase-negative, catalase-positive organisms 13. All the presumptive isolates fulfilling the above-mentioned characteristics were confirmed as Acinetobacter. For the selection of Carbapenem-resistant isolates, antibiotic susceptibility testing was performed on each isolate as recommended by Clinical & Laboratory Standards Institute (CLSI) guidelines. A lawn of bacterial inoculum of each isolate was made on MHA and was tested for the following antibiotics: ceftriaxone, co-trimoxazole, ofloxacin, meropenem, gentamicin, amikacin, and colistin. Isolates that were completely resistant to meropenem manifested by no zone of inhibition around the antibiotic disc were labeled as carbapenem-resistant and included in our study for the experiments.
At first, minimum inhibitory concentration (MIC) defined as the lowest concentration of an antibacterial agent that inhibits the visible growth of bacteria was determined for CrAgNPs using the broth macrodilution method on 5 isolates of CRA. Briefly, 0.5 McFarland turbidity suspension of each isolate of CRA was prepared in Brain Heart Infusion (BHI). Two-fold serial dilutions of CrAgNPs (0.01mg/ml, 0.02mg/ml, 0.04mg/ml, 0.08mg/ml, 0.16mg/ml)) were prepared in sterile tubes each containing 5ml of MHB. Each of the tubes was then inoculated with 50ul of bacterial suspension along with control. Tubes were incubated aerobically overnight at 37°C in the incubator and MIC was determined after 24 hours. The minimum concentration which inhibited the bacterial growth as appeared by the change in turbidity was noted as the MIC for CrAgNPs 14. This was further confirmed by plating 100µl of bacterial suspension from each tube on MH agar plates to observe complete inhibition of bacterial colonies at the respective MIC. Each experiment was conducted in triplicate.
For further evaluation of antibacterial activity, the agar well diffusion method was performed on 50 isolates of CRA. For this, 0.5McFarland suspension of each isolate was prepared in tryptone water, and 100µl of this was then plated on MHA plates and an 8mm well was made on it with sterile filter tips. Later, 100µl of CrAgNPs were poured into the wells, and plates were incubated aerobically for 24 hours at 37oC. The zone of inhibition was measured in millimeters 15. Colistin was used as a standard drug.
To appraise the combination of CrAgNPs and meropenem, a checkerboard assay was used which offers a precise estimation of synergistic, additive, or antagonistic types of drug interaction in vitro. The experiment was carried out in 12 separate tubes labeled from A-L each containing 1 ml of MHB and 15µl of bacterial suspension of CRA isolate. Two-fold serial dilutions of CrAgNPs ranging from 0.04 to 0.32mg/ml and meropenem ranging from 0.002-0.008mg/ml were prepared along the x-axis (abscissa) and y-axis (ordinate) respectively (Figure 1). Following incubation, a loopful of each tube was streaked on MH agar plates. The plates were then incubated at 37°C for 24 hours to observe growth. The earliest combination in the assay which first inhibited the growth was noted as the effective MIC for the combination and the fractional inhibitory concentration index (FICI) was calculated using the following formula:
FICA = MICA+B/MICA, FICB = MICB+A/MICB, FIC Index (FICI) = FICA+FICB
(≤0.5= Synergy, 0.5-4.0 additive or indifferent, > 4.0 antagonism) 16 17.
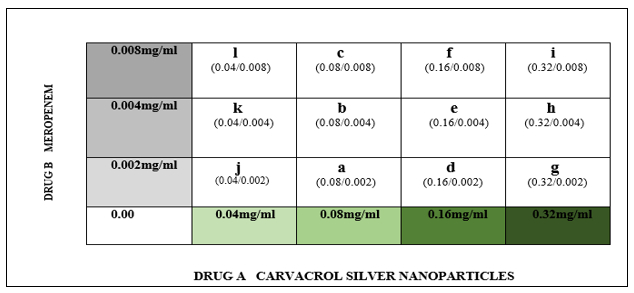
Figure 1: Conceptual Design of the Checkerboard Assay: The experiment was conducted in 12 tubes (represented by alphabets). Each tube had different concentrations of drug A and drug B in it along with MHB and bacterial inoculum. Green boxes show two-fold serial dilutions of drug A made in tubes along the x-axis while boxes in grey show two-fold serial dilutions of drug B made in tubes along the y-axis.
Results were analyzed using SPSS version 24. Numerical data was expressed in median and interquartile ranges. Kruskal-Wallis ANOVA with pair-wise comparison was applied to compare different drug groups. p-value less than 0.05 was considered statistically significant.
In our study, most of the samples were from the male patients. Adults aged 60 years or above were the most commonly affected age group followed by adults, neonates, adolescents, and infants. Most of the clinical isolates were cultured from tracheal secretions followed by pus, blood, wound, sputum, and CSF. Most of the bacterial isolates were collected from patients admitted in MICU with decreasing order of frequency being NICU, SICU, ward, and PICU (Table 1).
Table 1: Demographic data for the distribution of CRA isolates according to gender, age, source, and ward association.

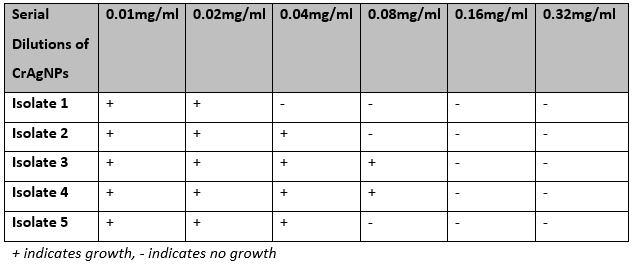
The MIC of CrAgNPs which was able to inhibit bacterial growth was found to be in the range of 0.04 to 0.16mg/ml (Table 2). For all further experiments, MIC was taken as 0.16mg/ml as this is the concentration at which the growth of all 5 isolates was inhibited. MIC was further confirmed by the absence of visible growth of bacteria on MHA plates at the corresponding MIC (Fig 2).
Table 2: Minimum Inhibitory Concentration (MIC) of CrAgNPs against five different carbapenem-resistant Acinetobacter isolates.
The agar well diffusion method was performed to observe the zone of inhibition of CrAgNPs at their corresponding MIC (Figure 2). Out of the 50 isolates, 11 isolates (22%) were resistant while 39 isolates (78%) were sensitive. The data for the zone of inhibition for 50 isolates is summarized in (Table 3A).

In the checkerboard assay, tube L was the first combination to completely inhibit bacterial growth so the FICI according to the mentioned formula was calculated to be 1.25 which demonstrates an additive effect of CrAgNPs and meropenem (Table 3B) (Figure 2).
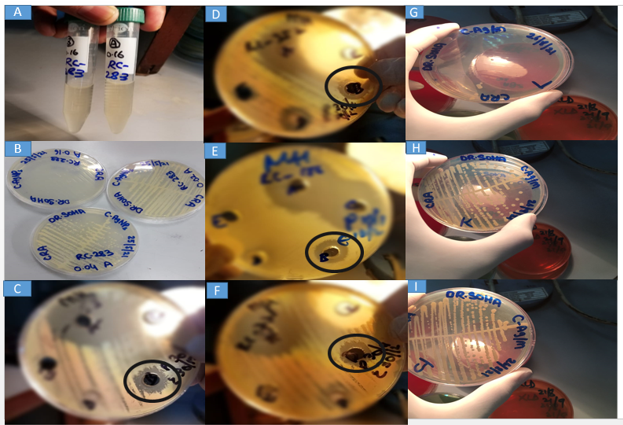
Figure 2: MIC, Agar Well Diffusion Method & Checker Board Assay.
A: Broth macrodilution method of MIC. B: MH agar plates showing growth patterns of a CRA isolate at different concentrations tested for MIC. C-F: MH agar plates showing zone of inhibition of CrAgNPs mentioned as B (encircled) in different CRA isolates. G: Plate labeled as L represents the first combination of drug A and drug B in the concentration of 0.04/0.008mg/ml in the checkerboard assay which inhibited bacterial growth. H & I: Plates labeled as J and K showed growth at concentrations 0.04/0.002mg/ml and 0.04/0.004mg/ml respectively in the checkerboard.
Acinetobacter is emerging as an important nosocomial pathogen in Pakistan though literature reporting their persistence is quite limited in our region 18. One arm of our study was intended to detect the range and distribution of CRA species among hospitalized patients in one of the tertiary care setups of Karachi. In our study majority of the clinical isolates of Acinetobacter were from male patients and this is in concordance with other studies conducted in many countries that also report a higher frequency of Acinetobacter infections in male gender 19,20. Patients 60 years and older were the most commonly affected group in our study which is a predictable finding as patients of older age are hospitalized more frequently for various medical conditions and are more likely to be exposed to nosocomial pathogens. The same pattern is also reported in many other studies 21, 22.
As these are renowned for causing ventilator-associated pneumonia, therefore trachea was the commonest source of these isolates. A larger study conducted in the US on Acinetobacter species also found the respiratory tract as a major culture source of these isolates 23. The majority of isolates were from samples of patients admitted to the intensive care unit with decreasing order of frequency being MICU, NICU & SICU. This is contrary to a finding reported by a study in Pakistan where the prevalence of infection was high in neonatal intensive unit care 24. Nevertheless, the weakened defense system of patients admitted to different intensive care units might be the plausible reason for these patients to become the victims of Acinetobacter species. In the scenario of antimicrobial resistance, it is inevitable to investigate new molecules that have the potential to eradicate such resistant organisms. In this regard, phytotherapy combined with nanotechnology is expected to upsurge in health sciences as it provides an opportunity to formulate novel drugs having safe, versatile, and proactive dynamics to fight the plight of antibacterial resistance 25. In line with this, we have explored the antibacterial activity of CrAgNPs on CRA isolates collected from a tertiary care setup in Karachi.
There is a lack of studies reporting the antibacterial activity of CrAgNPs therefore we have tested the MIC of CrAgNPs on five different isolates to corroborate the results of this novel compound which were further reinforced by performing agar well diffusion method on 50 isolates of CRA. Previous studies conducted on AgNPs have reported different MICs against Acinetobacter which are largely in disagreement with our findings. A study in Iran reporting the activity of AgNPs synthesized using Ferula asafetida reported MIC of 2µg/ml whereas another study conducted in Iraq where AgNPs were synthesized using the aqueous extract of chamomile flower reported MIC of 50µg/ml against Acinetobacter which could be attributed to different physiochemical properties of that nanoforms as well as different resistance profile of organism 26, 27. However, another study from Iraq on AgNPs produced using Myrtus communis leaves reported MIC somewhat closer to our finding which is 0.2mg/ml 28. On agar well diffusion we found the median zone of inhibition of around 11mm (0-20mm) which shows improved activity of CrAgNPs against Acinetobacter as compared to the standard drug colistin for which the ZOI is reported to be 14mm. Interestingly these particles also inhibit the growth of an isolate in the study resistant to colistin which is an amazing finding in the scenario of emerging pan-drug resistant isolates of this organism. A study performed on AgNPs of Sisymbrium irio in Saudi Arabia reported ZOI in the range of 11-21mm 29. A study conducted in South Korea on Areca catechu synthesized AgNPs reported ZOI against MDR A. baumannii to be in the range of 10.5-17.7mm 30.
In this study, we found additive interaction between CrAgNPs and Meropenem against Acinetobacter. This was contrary to the study between AgNPs and imipenem where MIC of the latter was significantly decreased against Acinetobacter baumannii (A. baumannii) depicting synergistic effects 31. Similar to our finding, an additive interaction between AgNPs and meropenem was also reported in a study on beta-lactamase-producing Klebsiella pneumoniae strains 32.
There are many plausible reasons for discrepant findings between different studies, foremost is the nature of plants or chemicals used for the synthesis of AgNPs which render their natural properties to the synthesized nanoformulations, and also each of these formulations varies in their physical properties such as size, shape, surface area, and morphology which indeed are an important criterion for the antibacterial activity of nanoformulations 33. Second but also noteworthy is that these studies were conducted on different strains of Acinetobacter causing infections in different regions across the globe, therefore they must have varied antibiotic resistance profiles. Further, some of the studies are specifically against the species A. baumannii of this genus.
The findings of this study should be generalized by conducting studies on a larger number of isolates collected from different regions of the world before further validation in-vivo. More sophisticated results can be produced if the experiments are performed at the level of Acinetobacter species, in particular, its most worrisome kindred A. baumannii. As this study is preliminary, therefore it would be injudicious to approximate the type of interaction between CrAgNPs and Meropenem which must be explored further by using a large number of samples and differently synthetized CrAgNPs having changed sizes and techniques thus having diverse physiochemical properties. Nevertheless, the utilization of nano metals particularly AgNps will be a cornerstone for the revival of safer drugs against impervious and obstinate microbes.
Carvacrol silver nanoparticles possess prospective antibacterial activity against carbapenem-resistant Acinetobacter which is quite encouraging in the wake of the urgent need for exploration of new drugs for resistant bacteria. Carvacrol silver nanoparticles have additive interaction with Meropenem pointing towards the possible restoration of carbapenems activity against this organism which may open future avenues in the formulation of older antibiotics with these molecules.
A special thanks to Ziauddin University for providing the grant for this study.
I declare that there are no conflicts of interest to disclose.
The research was funded by Ziauddin University, Pakistan.
The study is approved by the ethical review committee (ERC), Ziauddin University, Pakistan (Reference code: 2971220SHPHA)
SH, RS & ZA conceptualized the idea. RS & ZA synthesized nanoformulations. SH, AK, and FA carried out the experiments and interpreted the results. SH & KI wrote the manuscript. ZM supervised the project and proofread the manuscript.
- Rebic V, Masic N, Teskeredzic S, Aljicevic M, Abduzaimovic A, Rebic D. The importance of Acinetobacter species in the hospital environment. medical archives. 2018;72(5):325. doi: 10.5455/medarh.2018.72.330-334
- El-Sayed Ahmed MA, Zhong LL, Shen C, Yang Y, Doi Y, Tian GB. Colistin and its role in the Era of antibiotic resistance: an extended review (2000–2019). Emerging microbes & infections. 2020;9(1):868-885. doi: 10.1080/22221751.2020.1754133
- Iwu CD, Korsten L, Okoh AI. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen. 2020;9(9):e1035. doi.org/10.1002/mbo3.1035
- Sharifi‐Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, del Mar Contreras M, Salehi B, Soltani‐Nejad A, Rajabi S, Tajbakhsh M, Sharifi‐Rad J. Carvacrol and human health: A comprehensive review. Phytotherapy Research. 2018;32(9):1675-1687. doi.org./10.1002/ptr.6103
- Raei P, Pourlak T, Memar MY, Alizadeh N, Aghamali M, Zeinalzadeh E, Asgharzadeh M, Kafil HS. Thymol and carvacrol strongly inhibit biofilm formation and growth of carbapenemase-producing Gram negative bacilli. Cellular and Molecular Biology. 2017;63(5):108-112. doi.org/10.14715/cmb/2017.63.5.20
- Trevisan DA, Silva AF, Negri M, Abreu Filho BA, Machinski Junior M, Patussi EV, Campanerut-Sá PA, Mikcha JM. Antibacterial and antibiofilm activity of carvacrol against Salmonella enterica serotype Typhimurium. Brazilian Journal of Pharmaceutical Sciences. 2018;54. doi.org/10.1590/s2175-97902018000117229
- de Souza TA, Souza LR, Franchi LP. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicology and environmental safety. 2019; 171:691-700. doi.org/10.1016/j.ecoenv.2018.12.095
- Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. International journal of nanomedicine. 2020; 15:2555. doi.org/10.2147/IJN.S246764
- Ansari MA, Alzohairy MA. One-pot facile green synthesis of silver nanoparticles using seed extract of Phoenix dactylifera and their bactericidal potential against MRSA. Evidence-Based Complementary and Alternative Medicine. 2018;2018. doi.org/10.1155/2018/1860280
- Liao S, Zhang Y, Pan X, Zhu F, Jiang C, Liu Q, Cheng Z, Dai G, Wu G, Wang L, Chen L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. International journal of nanomedicine. 2019; 14:1469. doi.org/10.2147/IJN.S1N1340
- Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends in biotechnology. 2010;28(11):580-588. doi.org/10.1016/j.tibtech.2010.07.006
- Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Particle and fibre toxicology. 2014; 11:1-7. doi.org/10.1186/1743-8977-11-11
- Lal B, Vijayakumar S, Anandan S, Veeraraghavan B. Specimen Collection, Processing, Culture, and Biochemical Identification of Acinetobacter spp. InAcinetobacter baumannii 2019 (pp. 1-15). Humana Press, New York, NY. doi.org/10.1007/978-1-4939-9118-1_1
- Ibrahim S, Ahmad Z, Manzoor MZ, Mujahid M, Faheem Z, Adnan A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Scientific Reports. 2021;11(1):1-8. doi.org/10.1038/s41598-020-80805-0
- Feroze N, Arshad B, Younas M, Afridi MI, Saqib S, Ayaz A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microscopy Research and Technique. 2020;83(1):72-80. doi.org/10.1002/jemt.23390
- Yang SK, Yusoff K, Mai CW, Lim WM, Yap WS, Lim SH, Lai KS. Additivity vs. synergism: Investigation of the additive interaction of cinnamon bark oil and meropenem in combinatory therapy. Molecules. 2017;22(11):1733. doi.org/10.3390/molecules22111733
- Haque S, Ismail K, Khatoon A, Memon Z, Afridi FI, Shah R. Carvacrol Conjugated Zinc Oxide Nanoparticles as Prospective Agents against Clinically Isolated Carbapenem Resistant Acinetobacter Species. Med Forum 2022;33(9):33-37.
- ul Ain Q, Naim A, Saeed A. Prevalence and resistance profile of clinical isolates of Acinetobacter species from Karachi, Pakistan. RADS Journal of Biological Research & Applied Sciences. 2019;10(1):6-13. doi.org/10.37962/jbas.v10i1.163.
- Ayobami O, Willrich N, Suwono B, Eckmanns T, Markwart R. The epidemiology of carbapenem-non-susceptible Acinetobacter species in Europe: analysis of EARS-Net data from 2013 to 2017. Antimicrobial Resistance & Infection Control. 2020;9(1):1-10. doi.org/10.1186/s13756-020-00750-5.
- Mukhtar SY, Hassan MM, Elkhidir IM. Prevalence of Acinetobacter spp. in Intensive Care Units of Selective Hospitals at Khartoum State, Sudan. American Journal of Microbiological Research. 2022;10(1):1-5. doi:10.12691/ajmr-10-1-1.
- Zhao SY, Jiang DY, Xu PC, Zhang YK, Shi HF, Cao HL, Wu Q. An investigation of drug-resistant Acinetobacter baumannii infections in a comprehensive hospital of East China. Annals of clinical microbiology and antimicrobials. 2015;14(1):1-8. doi.org/10.1186/s12941-015-0066-4.
- Rebic V, Masic N, Teskeredzic S, Aljicevic M, Abduzaimovic A, Rebic D. The importance of Acinetobacter species in the hospital environment. medical archives. 2018;72(5):325. doi: 10.5455/medarh.2018.72.330-334.
- Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013–2017. BMC infectious diseases. 2019;19(1):1-9. Doi.org/10.1186/s12879-019-4387-3.
- Begum S, Hasan F, Hussain S, Shah AA. Prevalence of multi drug resistant Acinetobacter baumannii in the clinical samples from Tertiary Care Hospital in Islamabad, Pakistan. Pakistan journal of medical sciences. 2013;29(5):1253. doi: 10.12669/pjms.295.3695.
- Devi N, Rani K, Kharb P, Prasad M. Herbal medicine for urinary tract infections with the blazing nanotechnology. Journal of Nanoscience and Nanotechnology. 2021;21(6):3495-3512. doi.org/10.1166/jnn.2021.19002.
- Abootalebi SN, Mousavi SM, Hashemi SA, Shorafa E, Omidifar N, Gholami A. Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against Acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on the human cell lines. Journal of Nanomaterials. 2021;2021. doi.org/10.1155/2021/6676555.
- Mohamedsalih PM, Sabir DK. Biosynthesis of silver nanoparticles using the aqueous extract of chamomile flower and their antibacterial activity against Acinetobacter spp. Health Biotechnology and Biopharma. 2020;3(4):48-62.
- Sabir DK. Biosynthesized of Silver Nanoparticles from Myrtus communis Leaves and Investigates of their Antimicrobial Activities Against a Clinical Isolate of Acinetobacter baumannii. Acta Scientific Microbiology. 2022; 5:52-59. doi: 10.22034/HBB.2020.29.
- Mickymaray S. One-step synthesis of silver nanoparticles using Saudi Arabian desert seasonal plant Sisymbrium irio and antibacterial activity against multidrug-resistant bacterial strains. Biomolecules. 2019;9(11):662. doi.org/10.3390/biom9110662.
- Choi JS, Jung HC, Baek YJ, Kim BY, Lee MW, Kim HD, Kim SW. Antibacterial activity of green-synthesized silver nanoparticles using Areca catechu extract against antibiotic-resistant bacteria. Nanomaterials. 2021;11(1):205. doi.org/10.3390/nano11010205.
- Khaled JM, Alharbi NS, Siddiqi MZ, Alobaidi AS, Nauman K, Alahmedi S, Almazyed AO, Almosallam MA, Al Jurayyan AN. A synergic action of colistin, imipenem, and silver nanoparticles against pandrug-resistant Acinetobacter baumannii isolated from patients. Journal of Infection and Public Health. 2021;14(11):1679-1685. doi.org/10.1016/j.jiph.2021.09.015
- Panáček A, Smékalová M, Večeřová R, Bogdanová K, Röderová M, Kolář M, Kilianová M, Hradilová Š, Froning JP, Havrdová M, Prucek R. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids and Surfaces B: Biointerfaces. 2016; 142:392-399. doi.org/10.1016/j.colsurfb.2016.03.007.
- Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Advanced healthcare materials. 2018;7(13):1701503. doi.org/10.1002/adhm.201701503.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
