By Asma Abdul Razzak1, Mehwish Qamar2, Farzana Adnan3, Maria Qureshi4, Abdul Hameed Sheikh5
- Gastroenterology Department, Medicare Cardiac and General Hospital, Karachi, Pakistan
- Nephrology Department, SHED hospital, Karachi, Pakistan
- Nephrology Department, Liaquat National Hospital, Karachi, Pakistan
- Nephrology Department, Karachi Institute of Kidney Disease, Karachi, Pakistan
- Nephrology Department, Syed Abdullah Shah Institute of Medical and Sciences, Sehwan Sharif, Pakistan
DOI: https://doi.org/10.36283/PJMD13-1/012
How to cite: Razzak AA, Qamar M, Adnan F, Qureshi M, Sheikh AH. Predictors of Seroconversion of Hepatitis-C Virus in End-Stage Renal Disease Patients. Pak J Med Dent. 2024;13(1): 64-68. Doi: 10.36283/PJMD13-1/012
Background: Chronic kidney disease (CKD) is a significant health challenge globally. Hemodialysis, the primary treatment for CKD, not only sustains lives but also increases the risk of disease transmission, particularly hepatitis C (HCV), with a 48.9% seroconversion rate. This study aimed to identify factors contributing to HCV seroconversion of hemodialysis patients in Karachi.
Methods: A cross-sectional study was conducted at three dialysis units within tertiary care hospitals in Karachi, from June 2022 to June 2023. A total of 141 patients aged between 18 and 65, of any gender, undergoing chronic hemodialysis for a minimum of 6 months, demonstrating good adherence, and possessing comprehensive serological data for HCV (baseline test and follow-up screening) were included. Data was analyzed using SPSS version 27 and a comparison between baseline characteristics and risk factors with seroconversion of HCV was done using independent samples t-test/Chi-square test. A p<0.05 was considered statistically significant.
Results: The patients had a mean age of 33.30±8.04 years. Seroconversion occurred in 14.9% of cases, with 21% testing positive for HCV on PCR. Notably, a higher prevalence of blood transfusion history (p=0.002), surgery history (p=0.001), a family history of HCV (p=0.001), dialysis at multiple centers (p=0.001), and initiating dialysis with an arteriovenous fistula (p=0.015) was observed among HCV-positive patients who underwent seroconversion, in comparison to those who remained HCV-negative.
Conclusion: A history of blood transfusion and surgery, a family history of HCV, dialysis at multiple centers, and initiation of dialysis with arteriovenous fistula are notable predictors for seroconverted positive HCV patients.
Keywords: Arteriovenous fistula, Renal Dialysis, Hepatitis C.
Globally, chronic kidney disease (CKD) stands as the primary contributor to illness and death. In nations such as Pakistan, located in South Asia, there is a notable occurrence of CKD, ranging from 21.2% to 23.3%1-3. The incidence of CKD is on the rise in Pakistan due to several factors, including insufficient healthcare services, limited government funding, a lack of health education, a high prevalence of hypertension and diabetes in the general population, and dry weather conditions that increase the risk of renal stones and glomerulonephritis1-3. The primary treatment approach for these CKD patients in Pakistan involves hemodialysis (HD). Hemodialysis serves a dual purpose, sustaining the lives of CKD patients while also increasing the risk of disease transmission, including hepatitis C, hepatitis B, and HIV among these individuals1, 2, 4.
Hepatitis C (HCV) is a liver-affecting viral infection, and the term seroconversion denotes the shift from a negative to a positive status for anti-HCV antibodies following medical or surgical procedures, prominently observed in studies, especially among dialysis patients1-3, 5, 6. In the United Kingdom, the reported rate stands at 1.1%, while in Pakistan, it is notably elevated at 48.9%3, 7. Numerous risk factors contribute to HCV infection in HD patients, encompassing factors like younger age, duration of HD, number of blood transfusions, history of organ transplantation, dialysis mode, prevalence of HCV infection within the dialysis unit, and nosocomial transmission of HCV within HD units1, 2, 7-10.
In recent years, the rise in HCV seroconversion has emerged as a critical concern due to its association with elevated mortality rates, increased hospitalization, and a decline in the overall quality of life among HD patients. Consequently, the primary objective of the current study was to discern the factors responsible for HCV seroconversion in HD patients attending a tertiary care hospital in Karachi, Pakistan. This investigation was imperative for informing preventive measures, enabling early intervention, and enhancing the overall care of this vulnerable population.
This is a cross-sectional study, conducted across three dialysis units in tertiary care hospitals in Karachi, covering the period from June 2022 to June 2023. The sample size was determined using the Open Epi Sample Size calculator, taking into account a 15.6% seroprevalence of HCV associated with surgical intervention1, with a bound on the error of 6% and a confidence level of 95%. The calculated sample size was 141. Patient selection was based on the registry of each dialysis unit, recruiting 50 patients from each unit. Inclusion criteria encompassed individuals aged 18 to 65, of any gender, undergoing chronic hemodialysis for a minimum of 6 months with good adherence, and possessing comprehensive serological data for HCV, including baseline and follow-up screenings. Nine patients co-infected with hepatitis B and HIV were excluded. The non-random consecutive sampling technique was employed. The study received ethical approval from the institute’s ethical review committee (ERC#04890-2022), and informed consent was obtained from all eligible patients before initiating data collection.
HCV infection was defined by anti-HCV antibodies in sera, assessed through ELISA. Initial evaluations were conducted at the commencement of hemodialysis, and follow-up HCV serology was examined from medical records. Regular HCV screening occurred every 3 to 6 months for all chronic dialysis patients. Those with HCV seroconversion were assessed for factors such as dental procedures, duration of dialysis, family history of HCV, history of blood transfusion and surgery, multiple sexual partners, dialysis at multiple centers, and vascular access. Baseline information, including age and gender, was also recorded.
Data was analyzed using SPSS version 27. Mean and SD were computed for quantitative variables like age and duration of dialysis. Frequency and percentage were computed for gender, seroconversion, and risk factors. Comparison between baseline characteristics and risk factors with seroconversion of HCV was done using independent samples t-test/Chi-square test. A p-value<0.05 was considered statistically significant.
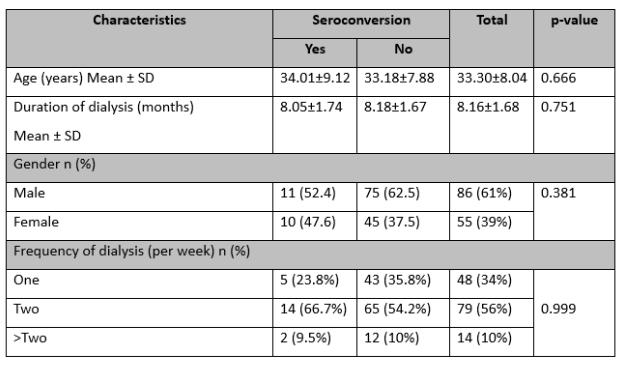
The patients’ mean age was 33.30±8.04 years, and the average duration of dialysis was 8.16±1.68 months. Out of 141 patients, 61% were male, and 39% were female. Approximately 56% of them underwent dialysis twice per week. The seroconversion rate was 14.9%, with 21% testing positive for HCV on PCR. There were no significant differences in age (p=0.666), duration of dialysis (p=0.751), frequency of dialysis (p=0.999), and gender (p=0.381) between patients with and without seroconverted HCV, as indicated in Table 1.
Table 1: Comparison of baseline characteristics between seropositive and seronegative HCV (n=141)
Among the 141 patients, 11.3% had a positive family history of HCV, 36.9% had a history of blood transfusion, 23.4% had a history of dental procedures, 22.7% had a history of surgery, 8.5% reported having multiple sexual partners, 19.1% underwent dialysis at multiple centers, and 74.5% initiated dialysis with an arteriovenous fistula.
Comparing patients with seroconverted positive HCV to HCV-negative patients, significantly higher proportions were observed in the former group for a history of blood transfusion (66.7% vs. 33.3%, p=0.002), a history of surgery (57.1% vs. 42.9%, p<0.001), a family history of HCV (61.9% vs. 38.1%, p<0.001), dialysis at multiple centers (66.7% vs. 33.3%, p<0.001), and initiation of dialysis with an arteriovenous fistula (95.2% vs. 4.8%, p=0.015), as outlined in Table 2.
Table 2: Comparison of seroconversion of HCV with risk factors (n=141)
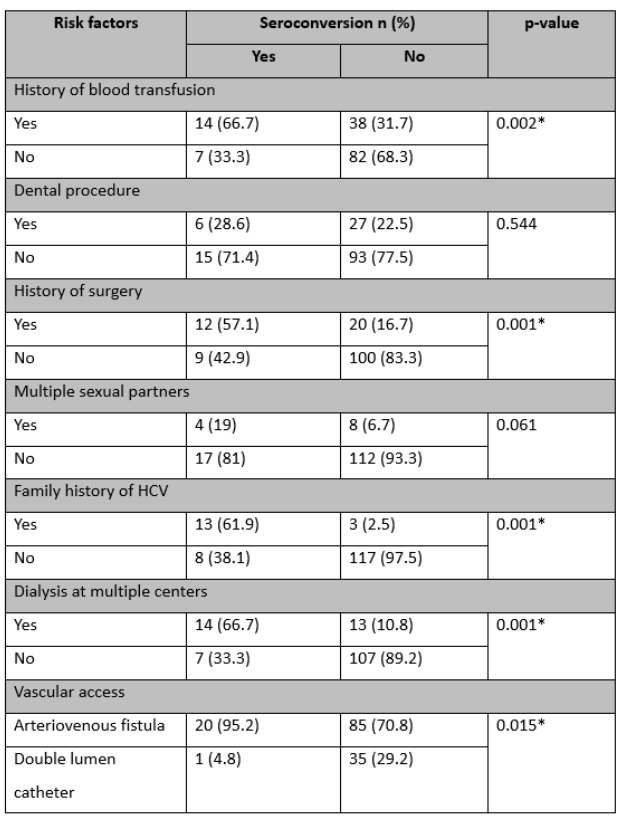
Data presented as n (%), *Significant at a 5% level of significance
In Pakistan, HCV infection is one of the most frequent diseases. It can result in Hepatocellular carcinoma and Decompensated Liver Disease2, 11-13. The risk of morbidity and mortality due to HCV is higher among patients with HD14. Fabrizi et al. conducted a meta-analysis and revealed that the risk of death is 1.6 times higher among HCV-positive patients15.
The primary objective of the current study was to predict factors contributing to seroconversion of HCV in HD patients. This phenomenon, where patients transition from negative to positive HCV antibody status after medical procedures like hemodialysis, has been a subject of interest in previous studies1, 2, 5, 16. Mostafa et al. found that the seroconversion rate of HCV was 14% in patients with ESRD and HCV infection17. In the current study, a seroconversion rate of 14.9%, is reported among HD patients presenting at three dialysis units in Karachi, Pakistan. The study also found that 21% of patients had HCV positive on PCR. Similarly, the study by Mahmud et al. conducted in Karachi found that seropositivity of HCV was 16.4% in HD patients18. Another study by Bukhari et al. conducted in Islamabad revealed that the seroconversion rate of HCV was 12.1% during the study period19. Another descriptive study carried out at Sheikhupura, Punjab by Hussaini et al. found a relatively higher proportion of seroconversion of HCV at 53.4% in chronic HD patients2. Sohail et al. undertook a systematic review and meta-analysis, determining a pooled prevalence of 32.33% for HCV among hemodialysis (HD) patients in Pakistan.20 This investigation indicates a notably elevated proportion of HCV in HD patients in the region of Punjab as compared to other regions of the country. Additionally, an examination of the effectiveness and safety of HCV management in HD patients demonstrated a 100% sustained virological response rate with the use of direct-acting antivirals21. These finding highlights the importance of regular screening and monitoring of HCV in HD units, as a significant portion of patients were already positive for the virus. Early detection is crucial to prevent further transmission and manage HCV infections effectively.
Numerous factors including duration of HD, attending multiple centers for HD, a history of invasive procedures, type of vascular access, dialyzer reuse, and frequency of blood transfusion were observed to be associated with HCV seroconversion1, 2, 22-24. In the study by Dharmesti et al., HCV seroconversion was found in 28% of the patients who were on regular dialysis, and HCV seroconversion was significantly associated with vascular access type (OR=42.1, 95% CI=5.8 to 307.4) and dialyzer reuse (OR=8.3, 95% CI=4.32-16.04)6., Mahupe et al. in their study found that HD duration, no. of hospital admissions, history of invasive procedures, and blood transfusions were not associated with seropositivity of HCV25. In the present study, we found history of blood transfusion and surgery, a family history of HCV, dialysis at different centers, and vascular access were significantly associated with HCV seroconversion. Hence, HCV seroconversion in end-stage renal disease patients on maintenance hemodialysis can lead to increased mortality, hospitalization, and reduced quality of life. Therefore, identifying the contributing factors is a critical step in prevention and early intervention. By understanding these factors, healthcare providers can implement targeted strategies to reduce the risk of seroconversion in this vulnerable population.
The current study has few limitations. Although efforts were made to calculate a representative sample size, the study’s relatively modest sample size might impact the generalizability of the results. Larger, multicenter studies could provide a more comprehensive understanding of the predictors of HCV seroconversion in hemodialysis patients. The exclusion of patients co-infected with hepatitis B and HIV might limit the study’s ability to explore potential interactions between these infections and HCV seroconversion. Future research considering these co-infections could provide a more comprehensive perspective. The study assessed specific risk factors such as the history of blood transfusion, surgery, family history of HCV, dialysis at multiple centers, and vascular access. However, other potential risk factors, such as socioeconomic status, comorbidities, and adherence to infection control measures, were not extensively explored.
The study findings revealed that specific risk factors significantly contribute to the seroconversion of HCV in this vulnerable population. A history of blood transfusion and surgery, a family history of HCV, dialysis at multiple centers, and initiation of dialysis with arteriovenous fistula emerged as notable predictors. Recognizing these factors is imperative for developing targeted preventive strategies, early interventions, and comprehensive care protocols.
None.
The authors declared no conflict of interest.
The study received ethical approval from the institute’s ethical review committee (ERC#04890-2022).
Informed consent was taken from the patients.
All authors contributed equally.
- Anees M, Sarwar N, Ahmad S, Elahii I, Mateen FE. Factors Associated with Seroconversion of Hepatitis C Virus in End Stage Renal Disease Patients. J Coll Physicians Surg Pak. 2021;31(9):1040-1045 DOI: 10.29271/jcpsp.2021.09.1040
- Hussain Y, Shahzad A, Azam S, Munawar N. Hepatitis-C and its seroconversion in end stage kidney disease patients on maintenance hemodialysis and factors affecting it. Pak J Med Sci. 2019;35(1):66-70 DOI: 10.12669/pjms.35.1.366
- Ismail T, Batool K, Abbasi Z, Khurshid T. Seroconversion of patients undergoing haemodialysis from HCV negative to HCV positive status. J Rawal Med Coll Stude Suppl. 2016;20(S-1):34-37
- Garthwaite E, Reddy V, Douthwaite S, Lines S, Tyerman K, Eccles J. Clinical practice guideline management of blood borne viruses within the haemodialysis unit. BMC nephrology. 2019;20:1-22
- Kerollos KMN, El-Ameen HA, El Wahed LA, Azoz NMA. Prevalence and seroconversion of hepatitis C among hemodialysis patients in Assiut governorate, Egypt. The Egyptian Journal of Internal Medicine. 2020;32(1):2 DOI: 10.1186/s43162-020-00005-0
- Dharmesti NWW, Wibawa IDN, Kandarini Y. Hepatitis C Seroconversion Remains High among Patients with Regular Hemodialysis: Study of Associated Risk Factors. Int J Hepatol. 2022;2022:8109977 DOI: 10.1155/2022/8109977
- Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65(6):2335-2342 DOI: 10.1111/j.1523-1755.2004.00649.x
- Madhavan A, Sachu A, Balakrishnan AK, Vasudevan A, Balakrishnan S, Vasudevapanicker J. Prevalence of hepatitis C among haemodialysis patients in a tertiary care hospital in south India. Iran J Microbiol. 2020;12(6):644-649 DOI: 10.18502/ijm.v12i6.5041
- Hinrichsen H, Leimenstoll G, Stegen G, Schrader H, Fölsch UR, Schmidt WE. Prevalence and risk factors of hepatitis C virus infection in haemodialysis patients: a multicentre study in 2796 patients. Gut. 2002;51(3):429-433 DOI: 10.1136/gut.51.3.429
- Nguyen DB, Bixler D, Patel PR. Transmission of hepatitis C virus in the dialysis setting and strategies for its prevention. Semin Dial. 2019;32(2):127-134 DOI: 10.1111/sdi.12761
- Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Digestive diseases and sciences. 2019;64:910-917
- Jafri W, Kamran M. Hepatocellular carcinoma in Asia: a challenging situation. Euroasian Journal of Hepato-Gastroenterology. 2019;9(1):27
- Aziz B, Nazar T, Akhlaq S. The frequency of occurrence of Hepatocellular Carcinoma after direct antiviral therapy in Hepatitis C virus patients. Pakistan journal of medical sciences. 2019;35(1):101
- Butt N, Anoshia, Khan MA, Akbar A. Effectiveness of Sofosbuvir and Daclatasvir in treatment of Hepatitis-C: An experience of tertiary care hospital in Karachi. Pak J Med Sci. 2021;37(7):2014-2019 DOI: 10.12669/pjms.37.7.4627
- Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: Effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther. 2004;20(11-12):1271-1277 DOI: 10.1111/j.1365-2036.2004.02290.x
- Xu M, Liu F, Zhao Q, Zhou Y, Zhuang Y, Ji M. Seroprevalence of hepatitis C virus in Jinan, China, 2008–2020. European Journal of Medical Research. 2023;28(1):112 DOI: 10.1186/s40001-023-01063-0
- Mostafi M, Jabin M, Chowdhury Z, Khondoker MU, Ali SM, Tamanna R, et al. The outcome of Daclatasvir and low dose Sofosbuvir therapy in end-stage renal disease patients with hepatitis C virus infection. Ukrainian Journal of Nephrology and Dialysis. 2020;0(2(66)) DOI: 10.31450/ukrjnd.2(66).2020.01
- Mahmud HM, Siddiqui M, Bashir B, Ali SF, Baloch AA, Masroor M. Hemodialysis patients profile at Dow University of Health Sciences, Karachi. Pakistan. Pak J Med Sci. 2014;30(6):1327-1330 DOI: 10.12669/pjms.306.5364
- Bukhari F, Khan A, Mehmood T. Predictors of HCV Seroconversion Among End-stage Renal Disease Patients in Hemodialysis Unit. Bulletin of Pharmaceutical Sciences Assiut. 2020;43(2):199-208
- Akhtar S, Nasir JA, Usman M, Sarwar A, Majeed R, Billah B. The prevalence of hepatitis C virus in hemodialysis patients in Pakistan: A systematic review and meta-analysis. PLoS One. 2020;15(5):e0232931 DOI: 10.1371/journal.pone.0232931
- Ali Khan R, Ali A, Khan AY. Response of Sofosbuvir and Daclatasvir combination in Chronic Hepatitis C with Hemodialysis Pakistani Patients: A Single Centre Study. Int. j. endorsing health sci. res. 2018;6(4):28-36.https://doi.org/10.29309/TPMJ/2020.27.12.4787
- Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney International. 2004;65(6):2335-2342 DOI: 10.1111/j.1523-1755.2004.00649.x
- Kataruka M, Gupta S, Ramchandran R, Singh M, Dhiman RK, Lal Gupta K. Incidence and Risk Factors for Hepatitis C Virus and Hepatitis B Virus Seroconversion in End-Stage Renal Failure Patients on Maintenance Hemodialysis. Journal of Clinical and Experimental Hepatology. 2020;10(4):316-321 DOI: https://doi.org/10.1016/j.jceh.2019.11.002
- Jadoul M, Bieber BA, Martin P, Akiba T, Nwankwo C, Arduino JM, et al. Prevalence, incidence, and risk factors for hepatitis C virus infection in hemodialysis patients. Kidney International. 2019;95(4):939-947 DOI: https://doi.org/10.1016/j.kint.2018.11.038
- Mahupe P, Molefe-Baikai OJ, Saleshando G, Rwegerera GM. Prevalence and risk factors for hepatitis b and c among end-stage renal disease patients on hemodialysis in Gaborone, Botswana. Niger J Clin Pract. 2021;24(1):81-88 DOI: 10.4103/njcp.njcp_464_19
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
