By Asma Noreen,1 Falak Abro,1 Sana Dawood,2 Mehtab Hussain,1 Sobia Shah Alam,3 Uzma Arshad1
- Pediatrics Department, Jinnah Medical and Dental College, Karachi, Pakistan
- Pediatrics Department, Liaquat National Hospital, Karachi, Pakistan
- Pediatrics Department, Lahore Medical & Dental College, Lahore, Pakistan
DOI: https://doi.org/10.36283/PJMD13-1/007
How to cite: Noreen A, Abro F, Dawood S, Hussain M, Alam SS, Arshad U. Neonatal Hematological Abnormalities Associated with Maternal Pre-eclampsia at Birth. Pak J Med Dent. 2024;13(1): 31-36. Doi: 10.36283/PJMD13-1/007
Background: Pre-eclampsia (PE) can cause hematological abnormalities that can further exacerbate the existing morbidity in newborns. This study aimed to investigate neonatal hematological abnormalities associated with maternal pre-eclampsia at the time of birth.
Methods: This cross-sectional was conducted from January 2023 to July 2023, involving 122 neonates born at the Department of Pediatric Medicine, Sohail Trust Hospital in Karachi, Pakistan. It analyzed neonates of either gender, aged from birth to 24 hours of life, and born to pre-eclamptic mothers, and their demographic details were recorded. At delivery, 2 ml of umbilical venous blood was collected by a trained pediatric nurse and then sent to the institutional laboratory. Hematological changes like leukopenia, thrombocytopenia, or neutropenia were noted. To assess the influence of various factors on hematological outcomes, chi-square tests were applied, with a significance threshold set at p<0.05.
Results: In a total of 122 neonates, the mean age was 8.14±6.49 hours. The mean gestational age was 38.87±3.15 weeks. There were 68 (55.7%) neonates who were male. Low birth weight (LBW) was less than 2500 grams in 45 (36.9%) newborns. Thrombocytopenia, leukopenia, and neutropenia were identified in 32 (26.2%), 25 (20.5%), and 23 (18.9%) neonates respectively. Stratification of leukopenia concerning study variables revealed that gestational age and LBW had a significant association (p=0.0036, p=0.0072) with leukopenia. Moreover, neutropenia also had an association with LBW (p<0.0001).
Conclusion: These findings underscore the importance of early hematological screening for neonates born to pre-eclamptic mothers to facilitate timely diagnosis and care.
Keywords: Hematologic Diseases, Neonates, Pre-eclampsia.
In 2020, around 2.4 million children worldwide lost their lives within the first month of being born. This equates to around 6,700 newborns dying daily, constituting 47% of all child deaths below the age of 5. This percentage has increased from 40% in 19901. The main contribution to this burden of mortality (over 65%) is from 10 countries (mostly Asian countries), and Pakistan stands in third place. Pakistan accounts for nearly 7% of global neonatal deaths2.
Pre-eclampsia (PE), which further leads to eclampsia, prevails in 2-10% of pregnant females and has varying occurrence rates internationally3. PE is believed to contribute to maternal and neonatal morbidity as well as mortality. It is estimated that underdeveloped countries have higher rates of PE (2.8% of live births) than developed countries (0.4%)4. The precise etiology of PE still needs to be investigated. Abnormal placentation, maternal immune response, genetic predisposition, and maternal vascular disease are some of the important factors that might contribute to PE5,6. Eclampsia, premature labor, and unintentional hemorrhage are acute consequences of PE7. PE has a significant impact on neonatal outcomes while different implications include low Apgar score, IUGR, low birth weight (LBW), intrauterine death (IUD), neonatal respiratory distress syndrome (RDS), and increased need for neonatal intensive care unit admission8,9.
Babies born to mothers having PE are more at risk of developing hematological abnormalities that can further exacerbate the existing morbidity10. Maternal PE can also cause thrombocytopenia in neonates, which is labeled when the platelet count falls below 150,000/µl11. Sivakumar et al reported that 22% of preeclamptic mothers gave birth to infants who had thrombocytopenia12. The literature describes neutropenia (ANC<500) as another common complication that develops in neonates with a 50% occurrence rate13. A study found neutropenia, leukopenia, and anemia to be present among 37%, 39%, and 28% of newborns to pre-eclamptic mothers respectively14.
Neonates born to PE mothers may present with huge variations in hematological changes. Not much local literature exists regarding details about the spectrum of hematological abnormalities among newborns of pre-eclamptic mothers. The findings of this study were thought to help us estimate the existing problems of hematological abnormalities among newborns of PE mothers that can further help us in improving the healthcare and outcome of these neonates. This study evaluated the hematological abnormalities at birth in neonates born to preeclamptic mothers.
This descriptive cross-sectional study was conducted at the Department of Pediatric Medicine, Sohail Trust Hospital, Karachi, Pakistan, from January to July 2023. The sample size of 122 was determined using the WHO sample size calculator, considering a 95% confidence level and an 8% margin of error based on an estimated frequency of neonatal leukopenia in infants born to mothers with PE14. Additionally, the study received prior approval from the Ethical Review Committee with protocol number 000239/22.
Inclusion criteria encompassed term and preterm neonates of any gender, within 24 hours of birth, delivered by mothers diagnosed with pre-eclampsia. Both primigravida and multigravida mothers were included, while neonates born to mothers with certain specified conditions were excluded, such as Rh incompatibility, diabetes mellitus, and other medical illnesses, or those who received drugs like aspirin or who had multiple pregnancies. Informed written consent was obtained from the parents or guardians of the neonates after a comprehensive briefing about the study’s purpose.
Data on neonatal demographics (age and birth weight), gestational age, and maternal parity were meticulously recorded. Umbilical venous blood samples were collected from the neonates immediately after delivery, and these samples were analyzed for hematological abnormalities, including thrombocytopenia, leukopenia, and neutropenia.
Statistical analysis employed SPSS version 26.0, presenting qualitative variables as frequencies and percentages, and quantitative variables as means with standard deviations. To assess the influence of various factors on hematological outcomes, chi-square tests were applied, with a significance threshold set at p < 0.05.
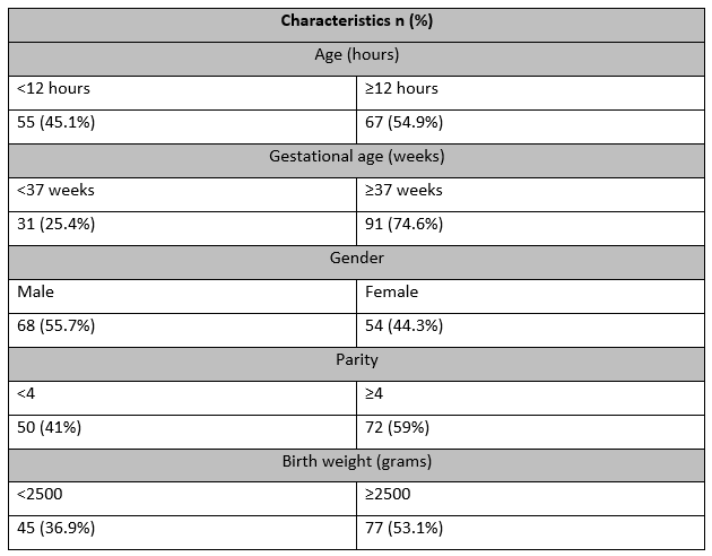
In this study, data from 122 neonates born to mothers diagnosed with pre-eclampsia with a mean age of 8.14±6.49 hours ranging between 2 to 24 hours. The mean gestational age was 38.87±3.15 weeks. There were 68 (55.7%) neonates who were male. Birth weight was less than 2500 grams in 45 (36.9%) newborns (Table 1).
Table 1: Baseline Characteristics of the Neonates Born to Preeclamptic Mothers.
Thrombocytopenia, leukopenia, and neutropenia were identified in 32 (26.2%), 25 (20.5%), and 23 (18.9%) neonates respectively (figure-1).
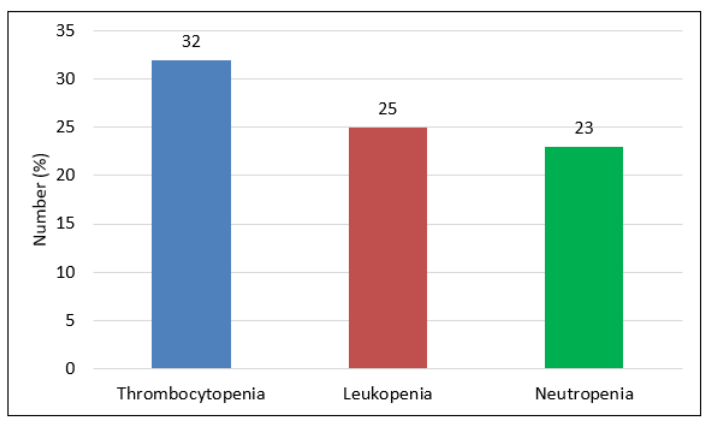
Figure 1: Frequency of Hematological Abnormalities among Neonates of Pre-eclamptic Mothers (n=122).
Stratification of study variables concerning thrombocytopenia showed that no statistically significant associations (p>0.05) were found. Stratification of leukopenia concerning study variables revealed that gestational age (p=0.0036) and LBW (p=0.0072) had having significant association with leukopenia. Stratification of neutropenia concerning study variables noted that LBW was associated with neutropenia (p<0.0001) as shown in Table 2.
Table 2: Stratification of thrombocytopenia concerning study variables (n=122)
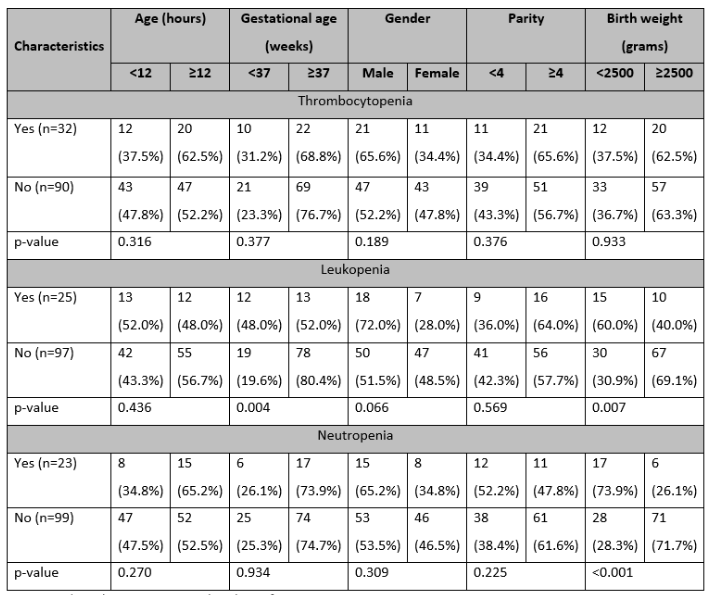
*p-value <0.05 is considered significant.
This research revealed the frequencies for thrombocytopenia, leukopenia, and neutropenia as 26.2% (25), 20.5% (25), and 18.9% (23) respectively. In a study from Qatar analyzing newborns of pre-eclamptic females, 13% of the newborns developed neonatal thrombocytopenia, which is lower than what we found in this study (26.2%)15. Our findings are closer to what Kiran et al found from India (22%)16. Harms et al noted the frequency of thrombocytopenia among newborns of pre-eclamptic mothers as 33% which is higher than what we found17. Relatively higher proportions of thrombocytopenia have been found by Eltink et al (34%), and Bhat et al (36%)18,19. The variations observed in these outcomes may be linked to several factors. One contributing factor could be the efforts made in the field of obstetrics to enhance the assessment and monitoring of women with a history of PE. Additionally, the economic and social status of diverse populations may also play a role in these differences.
The frequency of leukopenia was 20.5% in the present study. Bayoumi et al found the frequency of leukopenia among newborns of pre-eclamptic mothers as 11%. Our findings are very similar to what was noted by Harms et al (21%)17. In a prospective case-control study, preeclamptic mothers’ neonates were studied with an equal number of patients in both groups. In newborns of preeclamptic mothers, there was substantial increase in means of hemoglobin (17.6±1.17 vs. 14.6±0.65 g/dl, p<0.001), red blood cell count (4.96±0.32 vs. 4.56±0.22 x 106/mm3, p<0.001), mean corpuscular volume (108.9±4.7 vs. 104.76±1.93 fl, p<0.001), and reticulocyte count (10.75±2.52 vs. 2.04±0.87 %, p<0.001). Moreover, total leucocyte count (8989±818 vs. 15382±992 per mm3, p<0.001), mean neutrophil count (45.9±2.12 vs. 52.2±1.72, p<0.001), absolute neutrophil count (4723.15±255 vs. 9233±630, p<0.001), lymphocyte count (43.03±2.29 vs. 44.91±2.25 %, p<0.001), and platelet count (94.2±10.3 vs. 202.6±31.05 x103/mm3, p<0.001) showed significantly declined values20.
Another Nigerian study included 200 neonates, with 100 belonging to mothers with pregnancy-related hypertensive disorders and 100 belonging to normotensive mothers. A comparison showed that the neonates of normotensive moms had considerably lower mean hematocrit and higher neutrophil and platelet counts compared to the neonates of hypertensive mothers. The neonates of hypertensive mothers had polycythemia, neutropenia, and thrombocytopenia of 8%, 15%, and 38%, respectively, whereas the respective findings for the neonates of normotensive mothers were 0%, 2%, and 8%, showing a significant variance between the group findings21. These findings established that the frequency of hematological abnormalities among newborns of pre-eclamptic mothers is significantly high when compared to controls. Although newborns of hypertensive mothers show significant changes in their hematological profiles, these were not significantly related to rising neonatal morbidity as has been found by some researchers22.
PE is a maternal disease that affects the placenta and is linked to problems for the developing fetus23,24. The hematological profiles of babies born to preeclamptic mothers showed high abnormal proportions. Hematologic follow-ups can be done on these babies for monitoring and timely identification of the development of related abnormalities25. A relatively small sample size along with a single study center were some of the limitations of this study. We did not have any control groups so further local studies are needed to verify the findings of this study.
The frequency of thrombocytopenia, leucopenia, and neutropenia was found to be high in newborns of pre-eclamptic mothers. Early hematological screening of the newborns of pre-eclamptic mothers like complete blood count and peripheral smear examinations may help in the early diagnosis of hematological complications in these babies. There is a need for further research to validate these findings and improve neonatal healthcare outcomes.
The authors are thankful to M. Aamir Latif (RESnTEC) for his assistance in the statistical analysis of this research.
The authors declare no conflict of interest.
Ethical approval for conducting this study was obtained from the ethical review committee of the study institute (protocol number: 000239/22).
MAT is involved in the study conception, write-up, critical review, and approval of the final version of the manuscript. SI and ZM contributed to data collection, data analysis, and literature review.
- World Health Organization. Neonatal mortality. 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-mortality-report-2021
- Khan A, Kinney MV, Hazir T, Hafeez A, Wall SN, Ali N, et al. Newborn survival in Pakistan: a decade of change and future implications. Health Policy Plan. 2012;27(Suppl 3):iii72–iii87. doi: 10.1093/heapol/czs047
- Mou AD, Barman Z, Hasan M, Miah R, Hafsa JM, Trisha AD, et al. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep. 2021;11(1):21339. doi:10.1038/s41598-021-00839-w
- Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. J Pregnancy. 2011;2011. doi:10.1155/2011/481095
- Jung E, Romero R, Yeo L, Nardhy G, Piya C, Adithep J, et al. The etiology of preeclampsia. Am J Obstet Gynecol. 2022;226(2S):S844-S866. doi:10.1016/j.ajog.2021.11.1356
- Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J Clin Med. 2019;8(10):1625. doi:10.3390/jcm8101625
- Vigil-De Gracia P, Vargas C, Sánchez J, Collantes-Cubas J. Preeclampsia: Narrative review for clinical use. Heliyon. 2023;9(3):e14187. doi:10.1016/j.heliyon.2023.e14187
- Uzunov AV, Secara DC, Mehedințu C, Cîrstoiu MM. Preeclampsia and neonatal outcomes in adolescent and adult patients. J Med Life. 2022;15(12):1488-1492. doi:10.25122/jml-2022-0264
- Atamamen TF, Naing NN, Oyetunji JA, Wan-Arfah N. Systematic literature review on the neonatal outcome of preeclampsia. Pan Afr Med J. 2022;41:82. doi:10.11604/pamj.2022.41.82.31413
- Zhang N, Tan J, Yang H, Khalil RA. Comparative risks and predictors of preeclamptic pregnancy in the Eastern, Western and developing world. Biochem Pharmacol. 2020;182:114247. doi:10.1016/j.bcp.2020.114247
- Ceyhan T, Beyan C, Baser I, Kaptan K, Güngör S, Ifran A. The effect of preeclampsia on complete blood count, platelet count and mean platelet volume. Ann Hematol. 2006;85(5):320-322. doi:10.1007/s00277-006-0091-7
- Sivakumar S, Bhat BV, Badhe BA. Effect of pregnancy induced hypertension on mother and their babies. Indian J Pediatr. 2007;74:623-625. doi:10.1007/s12098-007-0110-2
- Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R. Effect of maternal hypertension on neonatal neutropenia and risk of nosocomial infection. Pediatrics. 1992; 90:430–435.
- Mosayebi Z, Nariman S, Hosseini L, Movahedian AH. Evaluation of laboratory disorders in admitted neonates in nicu who were born to preeclamptic mothers. J ComprPed. 2013;4(4):194-9. doi:10.17795/compreped-12755
- Bayoumi MAA, Ali AAH, Hamad SG, Ali AAM, Elmalik EE, Elkalaf MIR, et al. Effect of Maternal Preeclampsia on Hematological Profile of Newborns in Qatar. Biomed Res Int. 2020;2020:7953289. doi:10.1155/2020/7953289
- Kiran S, Bhaskar G, Jayashree N. A Study of Thrombocytopenia in Sick Neonates in a Tertiary Care Centre. Sch J App Med Sci, 2017;5(9B):3609-3616. doi: 10.36347/sjams.2017.v05i09.027
- Harms K., Rath W., Herting E., Kuhn W. Maternal hemolysis, elevated liver enzymes, low platelet count, and neonatal outcome. American Journal of Perinatology. 1995;12(1):1–6. doi: 10.1055/s-2007-994387
- Eeltink CM, van Lingen RA, Aarnoudse JG, Derks JB, Okken A. Maternal haemolysis, elevated liver enzymes and low platelets syndrome: specific problems in the newborn. European Journal of Pediatrics. 1993;152(2):160–163. doi: 10.1007/BF02072496.
- Bhat YR, Cherian CS. Neonatal thrombocytopenia associated with maternal pregnancy induced hypertension. Indian Journal of Pediatrics. 2008;75(6):571–573. doi: 10.1007/s12098-008-0110-x
- Mouna K, Doddagowda SM, Junjegowda K, Krishnamurthy L. Changes in Haematological Parameters in Newborns Born to Preeclamptic Mothers – A Case Control Study in a Rural Hospital. J Clin Diagn Res. 2017;11(7):EC26-EC29. doi:10.7860/JCDR/2017/29137.10303
- Okoye HC, Eweputanna LI, Korubo KI, Ejele OA. Effects of maternal hypertension on the neonatal haemogram in southern Nigeria: A case-control study. Malawi Med J. 2016;28(4):174-178. doi:10.4314/mmj.v28i4.5
- Tiruneh T, Kiros T, Getu S. Hematological reference intervals among full-term newborns in Ethiopia: a cross-sectional study. BMC Pediatr. 2020;20(1):417. doi:10.1186/s12887-020-02320-5
- Nelson KM, Irvin-Choy N, Hoffman MK, Gleghorn JP, Day ES. Diseases and conditions that impact maternal and fetal health and the potential for nanomedicine therapies. Adv Drug Deliv Rev. 2021;170:425-438. doi:10.1016/j.addr.2020.09.013
- Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies [published correction appears in Nat Rev Nephrol. 2019 Jun;15(6):386]. Nat Rev Nephrol. 2019;15(5):275-289. doi:10.1038/s41581-019-0119-6
- Dziegiel MH, Krog GR, Hansen AT, Olsen M, Lausen B, Norgaard LN, et al. Laboratory monitoring of mother, fetus, and newborn in hemolytic disease of fetus and newborn. Transfus Med Hemother. 2021;48(5):306-315. doi:10.1159/000518782
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
