By Sadaf Saeed1, Humayun Afridi1, Tariq Ali Khan1, Anhum Haroon Jadoon1, Afnan Rehman1, Somia Gul1
- Rehman College of Dentistry, Peshawar, Pakistan
DOI: https://doi.org/10.36283/PJMD13-1/010
How to cite: Saeed S, Afridi H, Khan TA, Jadoon AH, Rehman A, Gul S. Impact of Non-Surgical Periodontal Therapy on HbA1c Levels of Type 2 Diabetes at Khyber College of Dentistry, Peshawar: A Quasi-Experimental Study. Pak J Med Dent. 2024;13(1): 50-56. Doi: 10.36283/PJMD13-1/010
Background: Diabetes mellitus (DM) constitutes a spectrum of persistent metabolic disorders originating from irregular glucose metabolism due to insufficient insulin production, compromised insulin function, or a combination of the two, leading to elevated blood sugar levels. This study assessed the influence of non-surgical periodontal therapy on glycated hemoglobin (HbA1c) levels in individuals with Type 2 Diabetes.
Methods: This quasi-experimental study was conducted at Khyber College of Dentistry, Peshawar, with 40 participants diagnosed with Type 2 diabetes for more than 3 years and suffering from chronic periodontitis. Patients with Type 1 diabetes, pregnant women, and those with other medical complications were excluded. HbA1c levels were assessed at baseline, and non-surgical periodontal therapy was administered. Patients were educated and scheduled for a follow-up after 3 months when HbA1c levels were reassessed. A paired t-test was employed to compare HbA1c levels pre-and post-intervention.
Results: In this study, males were 29 (58%) whereas 21 (42%) females. The mean value of HbA1c was less post-treatment with non-surgical periodontal therapy (6.928±0.866%) than pretreatment (9.048±0.73%) and results were statistically significant (p<0.001). After receiving therapy, the HbA1c level was significantly reduced in both genders (p<0.001). Irrespective of smoking status, the reduction of HbA1c level was statistically significant (p=0.01).
Conclusion: Non-surgical periodontal treatment significantly reduces HbA1c levels, indicating a potentially positive impact on glycemic control in individuals with diabetes, which has enhanced the dentist’s role as a part of the health team, and timely interventions can significantly improve prognosis.
Keywords: Diabetes Mellitus, Glycated Hemoglobin, Nonsurgical Periodontal Debridement.
Diabetes mellitus (DM) constitutes a spectrum of persistent metabolic disorders originating from irregular glucose metabolism due to insufficient insulin production, compromised insulin function, or a combination of the two, leading to elevated blood sugar levels1. In 2013, diabetes affected approximately 382 million people globally, with projections foreseeing an increase to roughly 592 million by 20352. Recent findings underscore a correlation between inflammation of the periodontium and heightened blood sugar levels among individuals with type 2 DM3. It’s worth noting that the incidence of periodontal disease is more pronounced in those with inadequately controlled Type 2 DMs when compared to individuals without systemic conditions4, 5.
Regular non-surgical periodontal therapy has proven to be effective in lowering blood sugar levels, leading to a notable and statistically significant reduction of approximately 0.5 percentage points in glycated hemoglobin6-8. This effect of periodontal treatment on HbA1c levels is exemplified by an average decline of 0.36% in HbA1c after three months8.
Following the completion of dental treatment, Stewart et al. found that the intervention group had a decline in HbA1c levels from 9.5 to 7.6 (reflecting a decrease of 17.1%). Within this intervened group, reductions in HbA1c levels were noted: 7.6 to 6.6 to 7.70, and 10.3 to 8.4 (18%) for the diet, oral hypoglycemic, and insulin subdivisions respectively. Notably, noteworthy changes of statistical significance were identified among subjects in the intervention group who underwent oral hypoglycemic treatment and insulin treatment9.
Based on Madianos et al. research, the intervention group demonstrated a decline in HbA1c levels during the 3–4-month period, with the reduction ranging from -0.27% to -1.03%. At the 6-month post-intervention juncture, the HbA1c levels showcased a decrease varying from -0.02% (not statistically significant) to -1.18% (highly statistically significant, p < 0.001).10 Sadia et al.11 observed a potential reduction in HbA1c levels among individuals undergoing treatment for periodontal disease, implying effective management of diabetes. Conversely, the group that did not receive treatment showed either no alteration or an elevation in HbA1c levels. The initial average HbA1c for the periodontitis treatment cohort measured 7.67±0.64, whereas the untreated group exhibited an average of 6.95±0.34 (p=0.279). After 3 months, the average HbA1c for the periodontitis treatment group lowered to 6.8±0.62, while the untreated group had an average of 6.98±0.36 (p<0.001).
The rationale of this study was the scarcity of local data regarding the effects of Non-Surgical Periodontal Therapy on HbA1c levels among Type 2 diabetic patients, as periodontal health depicted variations in different populations due to ethnic, genetic, and environmental reasons. Previously literature has shown periodontal therapy helped to lower the risk of systemic complications in diabetic patients. This study enhanced the dentist’s role as a part of the health team and also helped the clinician to educate patients and allow them to timely interventions which had significantly improved prognosis. Good teamwork among dentists and physician lead to adequate therapy results and ultimately reduces diabetes complications. The objective was to assess the influence of non-surgical periodontal therapy on glycated hemoglobin (HbA1c) levels in individuals with Type 2 Diabetes.
The quasi-experimental study was conducted at Khyber College of Dentistry, Peshawar from 1st February 2021 to 31st July 2021 after the approval of the institutional ethics review board of Khyber College of Dentistry, Peshawar with reference number 1245/AD/PG/R/KCD/2019, by non-probability sampling. Written informed consent was obtained from all participants.
The participants included in the study were individuals with Type 2 diabetes, having a duration of more than 3 years (more is the cut–off for the duration of diabetes more are the chances for complications that’s why selected the midway from 3 years) and at least seven teeth per arch (to take data we need at least 7 teeth to be present in each arch for reference teeth) and at least seven teeth per arch. Exclusion criteria encompassed those diagnosed with type 1 diabetes, current smokers or those with a smoking history in the past 3 years, pregnant women, individuals taking medications that could affect study outcomes (such as calcium channel blockers, phenytoin, cyclosporine), and those with a history of periodontal therapy or periodontal diseases like gingivitis or periodontitis in the last six months (as determined by clinical examination).
By using Open Epi, the calculated sample size was 40 by taking baseline HbA1c of the treatment group (9.5±2.2) and after provision of Non-Surgical Periodontal therapy (7.6±1.4)4 among type 2 diabetic patients having chronic periodontitis by keeping 90% power of the test and 95% confidence interval to detect a mean difference of 1.94.
A comprehensive patient history was obtained, followed by an external examination. The initial intraoral clinical assessment for this research encompassed evaluating pocket depths (the measurement from the free gingival margin to the pocket’s base) and clinical attachment levels (the measurement from the cemento-enamel junction to the pocket’s base) at six specific sites (mid-buccal, mesio-buccal, disto-buccal, mesio-lingual, mid-lingual, disto-lingual) on each tooth, utilizing the Williams Periodontal Probe. The mean probing pocket depth will be calculated from these measurements (by dividing the number of sites probed by the number of teeth probed). Additionally, baseline HbA1c levels were recorded, and Non-Surgical Periodontal therapy was administered. Patient education was conducted, and follow-up appointments were scheduled for after 3 months.
Following the completion of active periodontal treatment, subsequent follow-up assessments at the three-month mark encompassed all components of the initial examination including the measurement of HbA1c levels and periodontal pocket depth only, no other assessments were done.
Data analysis was done in SPSS 22.0. Frequency and percentages were calculated for categorical variables like gender and smoking status. Mean and Standard Deviation were calculated for numerical variables like duration of diabetes and HbA1c. A paired samples t-test was applied to assess the mean difference of HbA1c before & after intervention. Effect modifiers like gender and smoking status, were stratified to check their effect on HbA1c before and after intervention by using an independent sample T-test. A p-value < 0.05 was taken as the significance level.
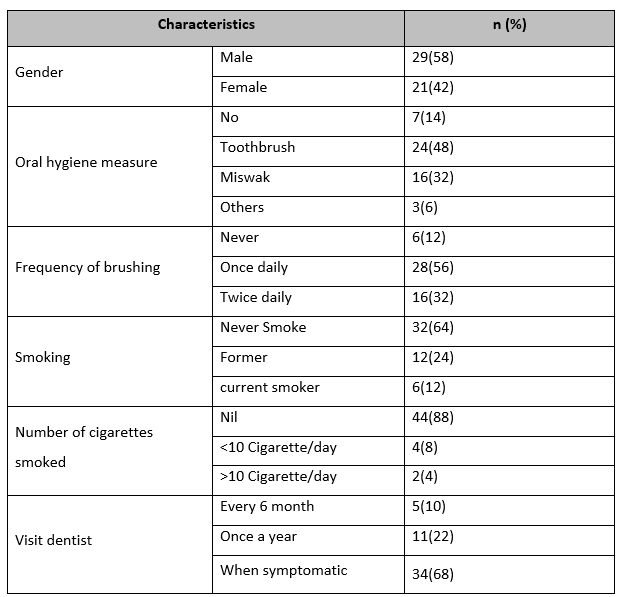
In this study, the males (n=29, 58%) were more than females (n=21, 42%). Toothbrush oral hygiene measure (n=24, 48%) was the commonest type followed by miswak (n=16, 32%). The most frequent tooth brushing frequency was once a day (n=28, 56%) followed by twice a day (n=16, 32%). Most of our sample was non-smokers (n=44, 88%) while four cases smoked less than 10 cigarettes/day and two subjects smoked more than 10 cigarettes/day. The most common pattern for visits to a dentist was when symptomatic (n=34, 68%). (Table 1)
Table 1: Frequency of gender, oral hygiene, brushing, smoking, number of cigarettes smoked, and visits dentist
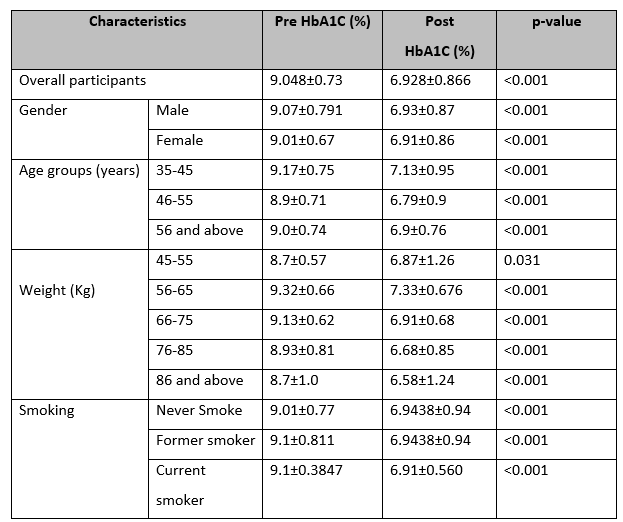
The participants in the study had an average age of 51.16 years, with a standard deviation of 8.33. The age range varied from 35 to 67 years. The average weight of the participants was 70.56 kg, with a standard deviation of 12.03. The weight ranged from 45 to 94 kg. The initial HbA1c levels (pre-intervention) had a mean of 9.048%, with a standard deviation of 0.73. The range of these levels was from 7.6% to 10.1%. After the intervention, the HbA1c levels (post-intervention) decreased, with a mean of 6.928% and a standard deviation of 0.866. The post-intervention HbA1c levels ranged from 5.3% to 8.6%. Eighteen participants were in the age group 46-55 years (36%) and above 55 years (36%) while the age group 35-45 years has 14(28%).
The mean value of HbA1C was less post-non-surgical periodontal therapy (6.928±0.866%) than pre-treatment (9.048±0.73%). These results were statistically significant (p<0.001). In both genders, the HbA1C level was reduced statistically after receiving non-surgical periodontal therapy (p<0.001). The post-surgical HbA1C level in males was 6.93±0.87% and in females was 6.91±0.86%. Similarly, the comparison of pre-and post-non-surgical periodontal therapy on HbA1C stratified by age groups shows that all HbA1c levels reduced after non-surgical periodontal treatment statistically significantly (p=0.01). Weight has no association with the results of our study. In all weight groups, the reduction in HbA1C after non-surgical periodontal therapy was very highly statistically significant (p<0.001). (Table 2)
Table 2: Comparison of pre- and post-non-surgical periodontal therapy on HbA1c stratified by demographic data.
*Paired t-test
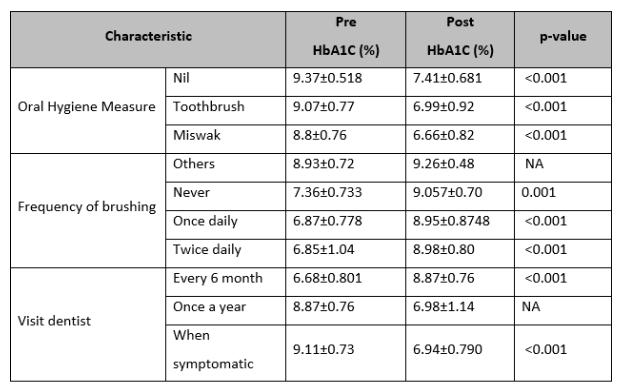
Smoking has no significant association for smoking with the effect of non-surgical periodontal therapy on HbA1C. Irrespective of smoking status (no, former, or current smoker) the reduction of HbA1C level was statistically significant (P<0.01). Similarly, no significant association between oral hygiene measures, frequency of brushing, and visits to a dentist with the effect of non-surgical periodontal therapy on HbA1C. (Table 3)
Table 3: Comparison of pre-and post-non-surgical periodontal therapy on HbA1C stratified by oral hygiene measures.
*Paired t-test
This study aimed to evaluate the impact of non-surgical periodontal therapy on HbA1c levels among Type 2 Diabetes patients receiving care at Khyber College of Dentistry in Peshawar, our study unveiled a remarkable and statistically significant reduction in HbA1c levels attributable to non-surgical periodontal treatment. In this study, we utilized HbA1c levels to record the glycemic level of the participants. HbA1c offers advantages over Fasting Blood Sugar (FBS) and Oral Glucose Tolerance tests (OGTT), including convenience, stability, and reduced stress-related fluctuations. However, these benefits must be weighed against higher costs, limited availability, and incomplete correlation with glucose levels. Long-term research shows a link between severe periodontitis, elevated HbA1c, and systemic issues in diabetes. Periodontitis may slightly elevate HbA1c in non-diabetics, potentially contributing to higher diabetes risk, but causality is not fully established3.
Recent research has suggested that periodontal infection might potentially disrupt the regulation of glycemic control by exacerbating insulin resistance. Consequently, non-surgical periodontal treatment, which disrupts the accumulation of bacterial plaque and addresses gingivitis, could potentially result in enhancements in glycemic levels9. This hypothesis gains support from studies that have observed a beneficial influence on diabetes sugar levels after periodontal intervention. However, it’s noteworthy that specific studies have not definitively established a direct cause-and-effect relationship, potentially due to insufficient time for the adequate healing of periodontal tissues or inadequate management of periodontitis. Furthermore, variables such as diet, physical activity, and the use of antidiabetic medications might significantly impact HbA1c levels, thereby complicating the ability to distinctly observe the metabolic effects of periodontal treatment4.
Numerous investigations have examined the impact of non-surgical periodontal therapy on blood sugar levels in individuals with diabetes. Both non-diabetic and diabetic patients experience similar short-term benefits as a result of non-surgical periodontal therapy, which encompasses reductions in pocket depth, enhancements in clinical attachment level (CAL), and modifications in subgingival microbiota5.
Liambés et al observed slight shifts in mean HbA1c, around 0.07%, which did not exhibit a statistically significant distinction after scaling in type 1 diabetic patients over a 3-month duration12. Similarly, Smith et al found that non-surgical periodontal therapy did not influence HbA1c levels in type 1 diabetic patients with inadequate control13. These similar conclusions were drawn from the Aldridge et al. study, which revealed no reduction in the level of HbA1c following periodontal therapy in type 1 diabetics suffering from severe periodontal loss14. Engebretsons et al. demonstrated that non-surgical periodontal therapy in type 2 diabetic patients with chronic periodontitis did not yield improvements in glycemic control for diabetes15. In consideration of these outcomes, the use of non-surgical periodontal treatment to decrease blood sugar levels may lack substantial justification. Gay et al in a randomized clinical trial involving 152 types 2 DM patients having periodontitis, found no statistically significant distinctions in the changes in HbA1c levels16.
In contrast, Farria-Almeida et al. showed a significant 5.7% decrease in HbA1c levels among type 2 diabetics using non-surgical periodontal therapy17. Dağ et al. and Auyeung et al. found significant HbA1c reduction with this therapy in well-controlled diabetics18,19. Recent systematic reviews have reported blood sugar improvements, with an average reduction of about 0.4% in HbA1c after non-surgical periodontal treatment13. Another study found an average -0.36% decrease in glycosylated HbA1c among type 2 diabetes patients15. A 1% HbA1c decrease is suggested to correlate with a 35% lower risk of microvascular complications20. A recent paper published in 2023 also found that periodontal intervention decreases blood glucose level 21.
The disagreement in the literature can be attributed to factors such as the patient’s genetics, the scale used to measure blood sugar, and the severity of diabetes mellitus. However, this is a study with a single center and a small sample size. Further studies with larger sample sizes and a multi-center case-control design are needed to better investigate this area. Additionally, these studies should consider the effects of hypoglycemic medications on HbA1c levels. It is important to note that this study does not include patients with well-controlled diabetes.
Based on our research findings, we can conclude that non-surgical periodontal treatments, such as scaling and root planning, lead to a significant and statistically proven reduction in HbA1c levels. Notably, the effectiveness of this intervention extends across diverse demographics, including both genders, smokers, non-smokers, all age groups, individuals of varying weight categories, and those with different frequencies of oral hygiene practices.
I would like to express my sincere gratitude towards my supervisor, Dr. Tariq Ali Khan, whose guidance, support, and insight went a long way in the completion of this article. I would like to thank all the faculty, the rest of my colleagues, and the staff at the Department of Periodontology and Implantology, Khyber College of Dentistry, Peshawar.
The authors declared no conflict of interest.
The ethical approval was taken from the Ethics Committee KCD with reference number 1245/AD/PG/R/KCD/2019.
SS made an Overall contribution to research and analysis. HA did Revision and corresponding authors, Data collection, and analysis. TAK did a critical review of the manuscript and expert research opinion. AHJ wrote the introduction. AR wrote a discussion. SG wrote the abstract.
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis : a tale of two common interrelated diseases. Nat Publ Gr. 2011;7(12):738–748. https://doi.org/10.1038/nrendo.2011.106
- Löe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. J Clin Periodontol 1986;13(5):431-440. https://doi.org/10.1111/j.1600-051X.1986.tb01487.x
- Milward M CI, Wright H, Millard J, Matthews J, Cooper P. Differential activation of NF-κB and gene expression in oral epithelial cells by periodontal pathogens. Clin Experim Immunol 2007;148(2):307-324. https://doi.org/10.1111/j.1365-2249.2007.03342.x
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007;78(7S):1387-1399 https://doi.org/10.1902/jop.2007.060264
- Holtfreter B, Albandar JM, Dietrich T, Dye BA, Eaton KA, Eke PI, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies. J Clin Periodontol 2015;42(5):407-412 https://doi.org/10.1111/jcpe.12392.
- Holtfreter B, Schwahn C, Biffar R, Kocher T. Epidemiology of periodontal diseases in the Study of Health in Pomerania. J Clin Periodontol 2009;36(2):114-123. https://doi.org/10.1111/j.1600-051X.2008.01361.x
- Genco RJ, Falkner KL, Grossi S, Dunford R, Trevisan M. Validity of self-reported measures for surveillance of periodontal disease in two western New York population-based studies. J Periodontol 2007;78(7S):1439-1454. https://doi.org/10.1902/jop.2007.060435
- Taylor GW, Borgnakke WS. Self-reported periodontal disease: validation in an epidemiological survey. J Periodontol 2007;78(7S):1407-1420. https://doi.org/10.1902/jop.2007.060481
- Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28(4):306-310. https://doi.org/10.1034/j.1600-051x.2001.028004306.x
- Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. 2018;45(2):188-195. https://doi.org/10.1111/jcpe.12836
- Salman S KK, Salman F, Hameed M. Effect of non-surgical periodontal treatment on glycemic control among type 2 diabetes mellitus patients with periodontitis. J Ayub Med Coll Abbott. 2016;28(4):742-745.
- Llambés F, Silvestre F-J, Hernández-Mijares A, Guiha R, Caffesse R. The effect of periodontal treatment on metabolic control of type 1 diabetes mellitus. Clin Oral Investigat. 2008;12(4):337-343. https://doi.org/10.1007/s00784-008-0201-0
- Smith GT, Greenbaum CJ, Johnson BD, Persson GR. Short-term responses to periodontal therapy in insulin-dependent diabetic patients. J Periodontol. 1996;67:794–802. https://doi.org/10.1902/jop.1996.67.8.794
- Aldridge JP, Lester V, Watts TL, Collins A, Viberti G, Wilson RF. Single-blind studies of the effects of improved periodontal health on metabolic control in type 1 diabetes mellitus. J Clin Periodontol. 1995;22:271–275. https://doi.org/10.1111/j.1600-051X.1995.tb00147.x
- Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, Seaquist ER, Reddy MS, Lewis CE, Oates TW, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310:2523–2532. doi:10.1001/jama.2013.282431
- Gay IC, Tran DT, Cavender AC, Weltman R, Chang J, Luckenbach E, Tribble GD. The effect of periodontal therapy on glycaemic control in a Hispanic population with type 2 diabetes: a randomized controlled trial. J Clin Periodontol. 2014;41:673–680. https://doi.org/10.1111/jcpe.12268
- Faria‐Almeida R, Navarro A, Bascones A. Clinical and metabolic changes after conventional treatment of type 2 diabetic patients with chronic periodontitis. J Periodontol. 2006;77(4):591-598. https://doi.org/10.1902/jop.2006.050084
- Dağ A, Firat ET, Arikan S, Kadiroğlu AK, Kaplan A. The effect of periodontal therapy on serum TNF-alpha and HbA1c levels in type 2 diabetic patients. Aust Dent J. 2009;54:17–22. https://doi.org/10.1111/j.1834-7819.2008.01083.x
- Auyeung L, Wang PW, Lin RT, Hsieh CJ, Lee PY, Zhuang RY, Chang HW. Evaluation of periodontal status and effectiveness of non-surgical treatment in patients with type 2 diabetes mellitus in Taiwan for 1 year. J Periodontol. 2012;83:621–628. https://doi.org/10.1902/jop.2011.110133
- Stratton IM AA, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J 2000;321(7258):405-412.
- Keller DC. HbA1c, and blood glucose, changes when treating periodontal disease with the perio protect method™. Oral Health Dent Sci. 2023;7(1):1-8.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
