By Ajet Kumar1, Syed Mahboob Alam2, Syeda Zain3, Saeeda Naseer Mengal4, Rozina Fazal5, Abdul Waheed Solangi6
1. Pharmacology Department, Ghulam Mohammad Mahar Medical College, Sukkur, Pakistan
2. Pharmacology Department, BMSI, JPMC, Karachi, Pakistan
3. Pharmacology Department, United Medical & Dental College, Karachi, Pakistan
4. Medicine Department, Jam Ghulam Qadir Govt Hospital, Hub, Pakistan
5. Medicine Department, District Head Quarter Hospital, Chaman, Pakistan
6. Pharmacology Department, JPMC, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD13-1/005
How to cite: Kumar A, Alam SM, Zain S, Mengal SN, Fazal R, Solangi AW. Exploring the Anti-Obesity Effects of Prebiotics and Probiotics in Rats; An Experimental Study. Pak J Med Dent. 2024;13(1): 17-23. Doi: 10.36283/PJMD13-1/005
Background: Excessive body fat, or obesity, poses a significant global health concern. Research has highlighted the connection between gut microbiota and obesity. This study aimed to investigate whether the administration of prebiotics and probiotics significantly contributes to controlling obesity in a rat model with induced obesity through a high-fat diet (HFD).
Methods: This experimental study involved 50 healthy male Albino Wistar Rats, aged between 10-14 weeks and weighing between 140-180 grams, whereas female rats and rats with any disease were excluded. They were divided into five groups: control (G-I), HFD alone (G-II), Prebiotic + HFD (G-III), Probiotics + HFD (G-IV), and Prebiotic + Probiotics + HFD (G-V). Data were analyzed using SPSS version 22, calculating Mean±S.D. ANOVA and post-hoc tests assessed weight changes between groups, with p-values ≤0.05 considered statistically significant.
Results: Weight changes were observed across all groups on days 0, 35, and 98. The percentages of mean weight change from day 0 to 98 were as follows: G-I, 11.08%; G-II, 47.98%; G-III, 29.88%; G-IV, 29.58%; and G-V, 20.54%. Significant differences (p<0.001) were noted among the groups.
Conclusion: Results in our study indicated that the inclusion of prebiotics and probiotics in the diet of rats following an HFD yields a beneficial outcome in terms of reduction of body weight. This positive effect is attributed to the stimulation and enhancement of the activity of beneficial gut bacteria. The synergistic interaction between prebiotics, probiotics, and the gut microbiota shows promise in mitigating obesity through targeted dietary interventions.
Keywords: Gastrointestinal Microbiome, Obesity, Prebiotic, Probiotics.
Obesity is a chronic metabolic disorder distinguished by an excessive accumulation of adipose tissue1. Among 400 million obese and 1.6 billion overweight individuals worldwide2, overweight prevalence in the United States is 66% and metabolic syndrome is 23% in 20 years and above and 40% in more than 60 years. In Pakistan prevalence of obesity among females is 10.2% and among males is 4.4% in the age group of 55 to 64 years. Meanwhile, the prevalence of overweight is 29.9% for females aged 35 to 44 years and 27.6% for males aged 45 to 54 years3.
Prebiotics are non-digestible compounds, often fibers that promote the growth and activity of beneficial bacteria in the gut. It remains indigestible in diet and in turn, has a targeted impact on the proliferation and function of a limited group of preexisting bacteria in the colon which contributes to improving the overall health of the host4. Prebiotics with a bifidogenic effect promote the growth of beneficial bacteria (Bifidobacterium & Lactobacillus) contribute to the fermentation of prebiotics, produce short-chain fatty acids (SCFAs), and create an environment that is less favorable for the growth of potentially harmful microorganisms by reducing their ratio (Bacteroidetes & Firmicutes)5,6. Probiotics comprise carbohydrates, including galacto-oligosaccharides, inulin, fructo-oligosaccharides, soybean oligosaccharides, cyclodextrins, gluco-oligosaccharides, xylo-oligosaccharides, lactulose and isomaltooligosaccharides, which resist digestion and proceed to the distal regions of the host’s digestive system to serve as nourishment for intestinal bacteria7.
New-generation probiotics (F. prausnitzii, A. muciniphila, P. Goldstein & Clostridia) are commonly found in most people’s microbiota; their reduction is associated with a higher risk of metabolic diseases and obesity8. A symbiotic interaction has been noted between the host and gut microbiota through the synergistic use of prebiotics and probiotics for addressing gastrointestinal disorders such as inflammatory bowel disease (IBD)9. This study aimed to investigate whether the administration of prebiotics and probiotics significantly contributes to controlling obesity in a rat model with induced obesity through a high-fat diet (HFD).
This experimental study took place at the Department of Pharmacology & Therapeutics, Basic Medical Sciences Institute (BMSI), Jinnah Postgraduate Medical Centre (JPMC) in Karachi, in collaboration with the Animal House at JPMC, following ethical approval from the Institutional Review Board (IRB -Ref. NO, F, 2-81/2020-GENL/39329/JPMC) of JPMC.
The study spanned a duration of six months, from March to August 2020. A total of 50 healthy male Albino Wistar Rats, weighing between 140-180 grams and aged between 10-14 weeks, were included in this study. Female rats, rats with any disease, and those rats who did not meet the inclusion criteria of age and weight were excluded from the study. The sample size was calculated by the parametric method of ANOVA for five groups10.
These rats were then randomly allocated into five groups, each consisting of an equal number, with 10 albino rats in each group (G). In this study, G-I, designated as the Control group, received a standard Chow diet exclusively. Meanwhile, G-II, G-III, G-IV, and G-V were subjected to a high-fat diet (HFD) regimen, with a daily intake of 200-250 grams over five weeks, inducing obesity. Following this initial 5-week obesity-inducing period, G-II continued with the HFD, G-III received a Prebiotic at a dosage of 140 milligrams per kilogram per day in addition to the HFD, G-IV received a Probiotic at a dosage of 1 billion colony-forming units per day alongside the HFD and G-V received both Prebiotic and Probiotic (Synbiotics) in combination with the HFD for an additional 9-week period.
Clean tap water was provided ad libitum to cages of all groups through a water bottle connected with an outlet tube. Inulin was employed as the prebiotic, while Bifidobacterium BB-12 and Lactobacillus rhamnosus GG served as the probiotics. These prebiotic and probiotic substances were administered to the rats using an oral gavage feeding tube in a normal saline medium. The rats were accommodated in appropriately marked stainless steel cages, and a consistent 12-hour cycle of light and darkness was maintained.
The data was systematically organized and subjected to analysis using the Statistical Package for the Social Sciences (SPSS) version 22 software. Mean values along with their respective Standard Deviations were computed for the recorded weights. To evaluate variations among the groups, an analysis of variance (ANOVA) was performed for multiple comparisons. A significance level of p < 0.05 was chosen to determine statistical significance.
On day 0, there were no statistically significant variations in mean weight among the groups (p=0.897). Figure 1 shows the effect of drugs on mean weight at days 0, 35, and 98. All values are expressed in gms.
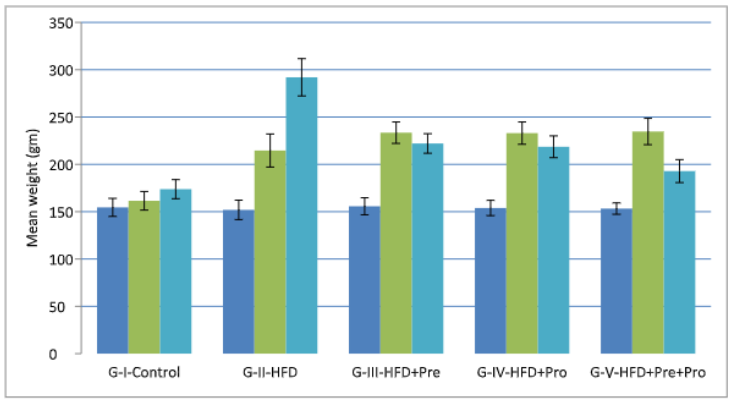
Figure 1: Effect of study drugs on mean weight (gms).
The initial mean weights at day 0 as shown in Table 1 were 154.54±9.52 in G-I, 151.90±10.24 in G-II, 155.72±8.99 in G-III, 153.92±8.09 in G-IV, and 153.26±6.02 in G-V. By day 35, significant differences emerged, with mean weights of 161.50±9.83 in G-I, 214.62±17.47 in G-II, 233.48±11.328 in G-III, 233.02±11.78 in G-IV, and 234.70±13.89 in G-V. Subsequently, on day 98, the mean weights were 173.80±10.10 in G-I, 292.0±19.71 in G-II, 222.08±10.33 in G-III, 218.58±11.52 in G-IV, and 192.88±12.13 in G-V, all demonstrating significant differences.
Table 1: Change in weight (gms) Mean±S.D at Day 0, 35, and 98.
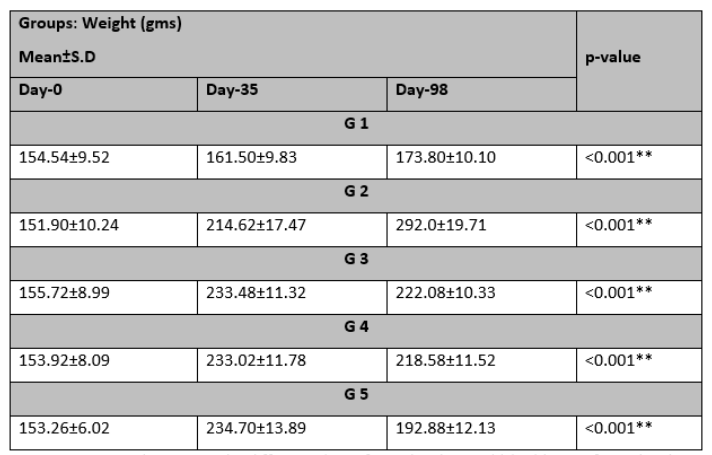 ANOVA was used to assess the difference*significant level <0.05** highly significant level <0.001
ANOVA was used to assess the difference*significant level <0.05** highly significant level <0.001
Table 2 provided a comprehensive overview of mean weight levels for different pairs of groups on days 0, 35, and 98. These results underscore the substantial (p<0.001) variations in mean weight within each group.
Table 2: Weight change (gms) Mean±S.D in different paired groups at Day 0, 35, and 98.
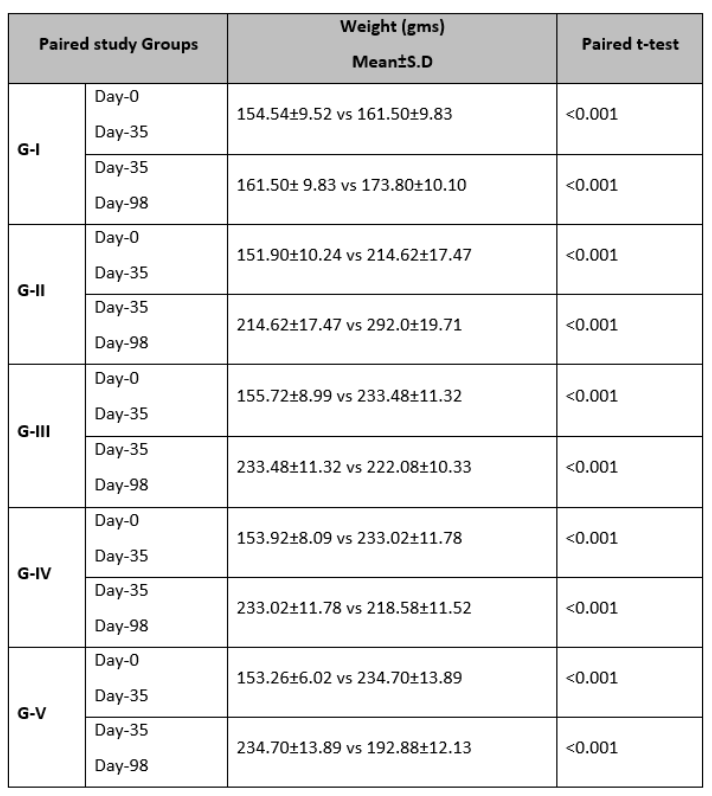
A paired t-test was utilized to evaluate the differences in paired groups, *p <0.05 statistically significant.
Table 3 showed changes in weight after introducing HFD at day 35 in all groups (G-I to V). Mean weight on day 0, revealed no significant differences among the groups. However, on day 35 and day 98, significant changes in mean weight levels were observed among the groups (p<0.001). Top of Form
Table 3: Change in mean weight (gms) in all Groups at days 0, 35, and 98.
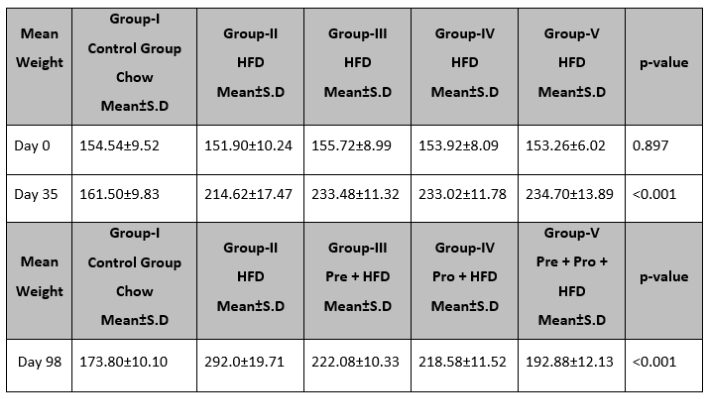
ANOVA was used to assess the difference, *p<0.05 is significant, HFD=High fat diet, Pre=Prebiotic, Pro=Probiotics, Chow=Chow Diet
Obesity is associated with an increased risk of several serious health conditions, including cancer, cardiovascular diseases, type 2 diabetes mellitus (T2DM), and liver diseases. The fundamental cause of obesity is an imbalance between caloric intake and energy expenditure. An imbalance in the composition of gut microbiota, known as gut dysbiosis, triggered by dietary or environmental shifts, can lead to the proliferation of pathogenic organisms. This, in turn, fosters chronic inflammation, significantly contributing to the development of chronic metabolic and intestinal disorders. Conversely, maintaining a healthy equilibrium of intestinal microbiota may serve as a preventive or alleviating function in the context of obesity and metabolic diseases11.
The gut microbiota, consisting of trillions of microorganisms like bacteria, has been implicated in the regulation of energy homeostasis. The fermentation of complex carbohydrates by gut bacteria, leading to the production of SCFAs, is one of the mechanisms through which the gut microbiota can influence energy metabolism. The SCFAs generated through microbial fermentation can be absorbed by the host and serve as a source of energy. Dysregulation of the gut microbiota has been associated with metabolic disorders, including obesity and its related health complications12.
Lifestyle factors, including diet, exercise, and the use of prebiotics and probiotics, are being explored as potential interventions to modulate the gut microbiota and improve metabolic health. The mechanisms by which probiotics influence body weight are not well understood. It seems that the primary mechanism of action is related to changing the composition and metabolites of intestinal microbiota13. In our study feeding an HFD for 5 weeks is responsible for the increase in body weight in rats and the consumption of prebiotics and probiotics till 14 weeks decreased the body weight. Prebiotics provide a fermentable food source that fosters the growth of beneficial gut microbiota (Bifidobacteria & Lactobacilli) 14.
The anti-obesity effects of probiotics are linked to the production of SCFAs, influencing appetite hormones, improving insulin sensitivity, and increasing energy expenditure. It also produces conjugated linoleic acid (CLA), which reduces body weight through enhanced lipid oxidation, adipocyte apoptosis, reduced lipogenesis, and anti-inflammatory actions15.
Probiotics contribute to gut health by enhancing the integrity of tight junctions between gut epithelial cells, thereby reducing intestinal permeability. This is significant as increased permeability can allow the translocation of bacteria and molecules across the intestinal barrier, potentially triggering inflammation. Probiotics help mitigate inflammation, particularly from lipopolysaccharides (LPS), known to be associated with inflammation when elevated in the blood. The reduction in inflammation leads to an increase in insulin sensitivity in the hypothalamus, which improves satiety. The increased concentrations of hormones such as leptin, glucagon-like peptide 1 (GLP-1), and pancreatic polypeptide (PPY) are potential outcomes of probiotic influence. These hormones play roles in appetite regulation and satiety, and their elevation could contribute to a reduction in food intake by promoting a sense of fullness16.
A study carried out on rats subjected to an HFD demonstrated a statistically significant rise in body weight when compared to the control group of rats, while on administration of yogurt supplements on HFD-fed rats, notably reduced their body weight17. In our study, the addition of prebiotics to an HFD in G-III resulted in a statistically significant decrease in weight by the end of day 98 (p<0.001). Our study’s findings are aligned with this research, demonstrating that rats in G-II, exclusively on an HFD, exhibited an increase in body weight (292.0±19.71gms) compared to rats in control G-I, (173.80±10.10 gms) at the end of 98-days, with a significant difference (p<0.001).
The effect of probiotics (Bifidobacterium longum, Lactobacillus helveticus, Lactococcus lactis Streptococcus thermophiles) with a prebiotic showed the beneficial effect on obesity in humans and on obese animals18,19. In our study addition of prebiotics to an HFD G-III could help counteract the negative effects of an HFD & and mitigate obesity. These changes enhance entero-endocrine cell function, improve glucose homeostasis, and increase leptin sensitivity in diabetic & and obese mice treated with oligofructose and other inulin-type fructans, and show lower levels of triglycerides, reduced adipose tissue accumulation, and decreased muscle lipid infiltration. Administration of short-chain fructo-oligosaccharides had beneficial effects on plasma lipid metabolism & and insulin levels20,21.
In our study, the addition of oligofructose into an HFD within G-III explains these effects on plasma lipid and blood insulin levels. Oligofructose supplementation is reported to prevent diet-induced obesity and glucose intolerance. It is known to improve host metabolism, including glucose, lipid, and energy metabolism22.
These metabolic improvements are attributed to changes in the gut microbiota composition and functional characteristics of the gut microbiota in obese rats. A combination of prebiotics and probiotics referred to as a symbiotic have the same properties of prebiotics and probiotics, together promoting probiotic survival in the gastrointestinal tract, and they might result in a better outcome regarding the host’s health when compared to prebiotics and probiotics separately23,24. The consumption of symbiotics affects the intestinal-brain axis. Highly fermentable prebiotics influenced the microbiota-elicited changes in GLP-1 and PYY and resulted in increased satiety, reduced hunger, and changes in appetite in both animals and humans 25.
The limitation of our study is the lack of human trials and the long-term effect of combined prebiotic and probiotic supplementation on gut microbiota and investigating the variability in individual responses against more bacterial strains.
The synergistic incorporation of prebiotics and probiotics into a high-fat diet effectively mitigates weight gain by influencing energy metabolism, appetite regulation, and nutrient absorption. This combined approach controls negative effects, enhancing insulin sensitivity, reducing inflammation, and ameliorating dyslipidemia, key contributors to obesity-related complications. Crucially, the modulation of gut microbiota emerges as a pivotal factor, mediating observed improvements in metabolic health and weight regulation. The findings suggest a promising strategy for obesity management, emphasizing the interconnected role of prebiotics, probiotics, and gut microbiota in mitigating the adverse impacts of a high-fat diet on body weight and metabolic well-being.
I would like to extend my heartfelt gratitude and recognition to the in-charge and staff of the animal house of BMSI, JPMC Karachi for their contributions to this research.
I declare that there are no conflicts of interest to disclose.
Ethical approval was taken from the Institutional Review Board (IRB -Ref. NO, F, 2-81/2020-GENL/39329/JPMC) of JPMC.
AK conceptualized the study, designed the research plan, acquired data through meticulous examination, and made significant contributions to manuscript writing. SMA provided overall supervision, contributing to the design, data interpretation, and critical manuscript reviews. SZ drafted and revised the manuscript, focusing on data interpretation. SNM contributed to data acquisition and manuscript writing. RF assisted in data collection and manuscript writing. AWS conducted critical analysis and contributed to manuscript writing. The collaborative efforts highlight the diverse roles, emphasizing a comprehensive approach to the study.
- León Aguilera XE, Manzano A, Pirela D, Bermúdez V. Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. J Pers Med. 2022;12(8):1282. doi: 10.3390/jpm12081282.
- Wiciński M, Gębalski J, Gołębiewski J, Malinowski B. Probiotics for the Treatment of Overweight and Obesity in Humans-A Review of Clinical Trials. Microorganisms. 2020;8(8):1148. doi: 10.3390/microorganisms8081148.
- Asif M, Aslam M, Altaf S, Atif S, Majid A. Prevalence and Sociodemographic Factors of Overweight and Obesity among Pakistani Adults. J Obes Metab Syndr. 2020;29(1):58-66. doi: 10.7570/jomes19039.
- Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687-701. doi: 10.1038/s41575-020-0344-2.
- You S, Ma Y, Yan B, Pei W, Wu Q, Ding C, Huang C. The promotion mechanism of prebiotics for probiotics: A review. Front Nutr. 2022;9:1000517. doi: 10.3389/fnut.2022.1000517.
- Noor J, Chaudhry A, Batool S, Noor R, Fatima G. Exploring the Impact of the Gut Microbiome on Obesity and Weight Loss: A Review Article. Cureus. 2023;15(6):e40948. doi: 10.7759/cureus.40948.
- Barathikannan K, Chelliah R, Rubab M, Daliri EB, Elahi F, Kim DH, Agastian P, Oh SY, Oh DH. Gut Microbiome Modulation Based on Probiotic Application for Anti-Obesity: A Review on Efficacy and Validation. Microorganisms. 2019;7(10):456. doi: 10.3390/microorganisms7100456.
- Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF, Lu CC, Lai HC. Next-generation probiotics in disease amelioration. J Food Drug Anal. 2019;27(3):615-622. doi: 10.1016/j.jfda.2018.12.011.
- Roy S, Dhaneshwar S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J Gastroenterol. 2023; 29(14):2078-2100. doi: 10.3748/wjg.v29.i14.2078. Erratum in: World J Gastroenterol. 2023;29(35):5178-5179.
- Ko MJ and Lim CY. General considerations for sample size estimation in animal study. Korean J Anesthesiol.2021;74(1):23-29. https://doi.org/10.4097/kja.20662.
- Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020;25(2):113-123. doi: 10.3746/pnf.2020.25.2.113.
- Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, Khalil M, Wang DQ, Sperandio M, Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci. 2022;23(3):1105. doi: 10.3390/ijms23031105.
- Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity-related diseases, Biomedicine & Pharmacotherapy.2022;147:112678, ISSN 0753-3322, https://doi.org/10.1016/j.biopha.2022.112678.
- Kaur H, Kaur G, Ali SA. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation. 2022; 8(9):425. https://doi.org/10.3390/fermentation8090425
- Soltani S, Ashoori M, Dehghani F, Meshkini F, Clayton ZS, Abdollahi S. Effects of probiotic/synbiotic supplementation on body weight in patients with diabetes: a systematic review and meta-analyses of randomized controlled trials. BMC Endocr Disord. 2023;23(1):86. doi: 10.1186/s12902-023-01338-x.
- Álvarez-Arraño V, Martín-Peláez S. Effects of Probiotics and Synbiotics on Weight Loss in Subjects with Overweight or Obesity: A Systhematic Review. Nutrients. 2021;13(10):3627. doi: 10.3390/nu13103627.
- Lasker S, Rahman MM, Parvez F, Zamila M, Miah P, Nahar K, Kabir F, Sharmin SB, Subhan N, Ahsan GU, Alam MA. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci Rep. 2019;9(1):20026. doi: 10.1038/s41598-019-56538-0.
- Ben Othman R, Ben Amor N, Mahjoub F, Berriche O, El Ghali C, Gamoudi A, Jamoussi H. A clinical trial about effects of prebiotic and probiotic supplementation on weight loss, psychological profile, and metabolic parameters in obese subjects. Endocrinol Diabetes Metab. 2023;6(2):e402. doi: 10.1002/edm2.402.
- Chen J, Chen X, Ho CL. Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Front Bioeng Biotechnol. 2021;9:770248. doi: 10.3389/fbioe.2021.770248.
- Weninger, S.N., Herman, C., Meyer, R.K. et al. Oligofructose improves small intestinal lipid-sensing mechanisms via alterations to the small intestinal microbiota. Microbiome.2023; 11(169). https://doi.org/10.1186/s40168-023-01590-2
- Wang B, Wang L, Wang H, Dai H, Lu X, Lee Y-K, Gu Z, Zhao J, Zhang H, Chen W, Wang G. Targeting the Gut Microbiota for Remediating Obesity and Related Metabolic Disorders.
The Journal of Nutrition.2021;151(7):1703-1716. https://doi.org/10.1093/jn/nxab103.
- Paone P, Suriano F, Jian C, Korpela K, Delzenne NM, Van Hul M, Salonen A, Cani PD. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes. 2022;14(1):2152307. doi: 10.1080/19490976.2022.2152307.
- Chaiyasut C, Sivamaruthi BS, Kesika P, Khongtan S, Khampithum N, Thangaleela S, Peerajan S, Bumrungpert A, Chaiyasut K, Sirilun S, Sittiprapaporn P. Synbiotic Supplementation Improves Obesity Index and Metabolic Biomarkers in Thai Obese Adults: A Randomized Clinical Trial. Foods. 2021;10(7):1580. doi: 10.3390/foods10071580.
- Borka Balas R, Meliț LE, Lupu A, Lupu VV, Mărginean CO. Prebiotics, Probiotics, and Synbiotics-A Research Hotspot for Pediatric Obesity. Microorganisms. 2023;11(11):2651. doi: 10.3390/microorganisms11112651.
- Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, Jacka F, Dinan TG, Cryan JF. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv Nutr. 2021;12(4):1239-1285. doi: 10.1093/advances/nmaa181.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
