By Hamid Raza Gulzar1, Nosheen Zahoor2, Ambreen Zahoor3, Hina Saghir3, Mariam Tariq3, Asma Bibi3
1. Medicine Department, Aneurin Bevan University Health Board, NHS Wales, UK
2. Rheumatology Department, Aneurin Bevan University Health Board, NHS Wales, UK
3. Hazrat Bari Sarkar Medical and Dental College and Hospital, Islamabad, Pakistan
DOI: https://doi.org/10.36283/PJMD13-1/003
How to cite:Gulzar HR, Zahoor N, Zahoor A, Saghir H, Tariq M, Bibi A. Comparison of the Efficacy of Duloxetine Versus Pregabalin for Pain Relief of Neuropathy in Diabetics. Pak J Med Dent. 2024;13(1): 5-10. Doi: 10.36283/PJMD13-1/003
Background: Neuropathy is a common complication in diabetic patients with clinical manifestations of feet and hands paresthesia, pain in the lower legs, and a burning sensation in the soles. A wide variety of medications are used for diabetic neuropathy with varying degrees of pain relief reported. The study objective was to compare the efficacy of duloxetine versus pregabalin for pain relief of neuropathy in diabetics.
Methods: The study was a six-week, single-blind, Randomized Controlled Trial conducted at HBS Medical and Dental College and Hospital in Islamabad. Patients were randomly designated to either of the groups (A or B) with 50 participants in each group. Group A received 150mg of pregabalin twice a day while group B received 60mg of duloxetine once a day. Pain relief was the primary outcome which was considered as a ≥ 50% decrease in pain score on the Visual Analog Scale at 6 weeks from baseline.
Results: A total of 100 diabetic patients were registered in the study. The mean age in groups A&B was 48.36 and 50.56 (SD± 6.64) years respectively. The majority of the study population were males 39 (78%) and 42 (84%) in both groups. A total of 66% (n=33) in Group A and 74% (n=37) of the patients in Group B achieved pain relief. A comparison of the pain relief achieved between the two groups showed no statistical significance (p-value 0.383).
Conclusion: The study revealed that duloxetine and pregabalin are both efficacious in terms of pain relief for diabetic neuropathy in our population.
Keywords: Diabetes Mellitus, Diabetic Neuropathy, Duloxetine Hydrochloride, Pregabalin.
Diabetic neuropathy is the most common of the various complications of diabetes, affecting approximately 30% of people with diabetes1. Clinical manifestations include paresthesia and pain in the lower limbs (mostly feet) and rarely the hands. The pain is dull, achy, and worsens at night. Symptoms also include burning sensations in the soles of the feet and wide-based abnormal gait2.
In diabetics, the pathogenesis of pain is multifactorial. It takes into account metabolic derangements such as hyperglycemia, impaired glucose tolerance, dyslipidemia, oxidative and nitrosative stress, growth factor deficiencies, microvascular insufficiency, and autoimmune damage to nerve fibers3. In routine practice, the diagnosis of neuropathy related to diabetes is established on history and examination4. The primary aim of the pharmacological intervention is to achieve pain relief 5. Older agents like the tricyclic anti-depressant amitriptyline have been used for many years while recent times have seen the advent of innovative groups like SNRI (Serotonin and Norepinephrine reuptake inhibitors) (duloxetine) or anticonvulsants (pregabalin, gabapentin)6. Despite the options, one of the main deficiencies in the management of diabetic neuropathy is the relative dearth of comparative research.
Duloxetine belongs to the group serotonin and norepinephrine reuptake inhibitors (SNRI). It is permitted by the US Food and Drug Administration (FDA) for the management of painful neuropathy associated with diabetes at a dose of 60 mg once daily7. Effective pain relief was achieved in 59% of patients treated with duloxetine in one clinical trial8. Duloxetine is recommended by NICE (National Institute of Health and Care Excellence) guidelines as a first-line treatment for painful neuropathy associated with diabetes and pregabalin as a second-line agent9. Pregabalin is also FDA-approved for diabetic neuropathy10. It is an anticonvulsant that binds to the α2-δ subunit of the Ca++ channel and relieves pain by reducing the discharge of norepinephrine and substance P. In one study comparing pregabalin in a dosage of 300 mg OD (Once daily) with placebo, pain relief was seen in 29.1% of patients11. American Academy of Neurology considers pregabalin as the first line and all other treatment options as a second line12.
There is inadequate data on the efficacy of duloxetine and pregabalin in residents. Moreover, to our knowledge, no study has compared the two agents directly in our settings. The purpose of this study was to compare the effectiveness of duloxetine with pregabalin for pain relief of neuropathy in diabetics so that the preferable treatment for this common disorder could be highlighted.
Our study which was a six-month, single-blind, RCT was carried out at the Diabetic Clinic of HBS (Hazrat Bari Sarkar) Medical and Dental College and Hospital Islamabad from 15 January 2023 to 15 July 2023 after ethical approval from the hospital’s ethical review committee with reference number EC18/169,15thOCT 2022. The study enrolled patients aged between 18-65 years of either gender, who had diabetes for at least five years and clinical manifestations of neuropathy for the last six months. Neuropathy was diagnosed based on history and examination. These included symptoms of pain, numbness, paresthesia, tingling, and burning in the feet and/or hands. Patients with co-morbid neurological conditions, critical medical conditions as well as pregnant and lactating females were excluded.
Randomization was done by lottery method and patients were allocated either to Group A or Group B (pregabalin or duloxetine respectively). Group A was prescribed 150mg of pregabalin twice daily and Group B was given 60mg of duloxetine once a day. Subjects were seen for a minimum of 3 visits: an initial enrollment visit (screening and randomization) and scheduled visits at the 3rd and 6th week of treatment. Visual Analog Scale (VAS) was utilized to track pain. It is a validated, subjective scale to measure pain where scores are documented by noting on a 10 cm line which denotes a scale starting from “0 as no pain” to “10 as worst pain”. VAS scores were recorded at baseline and then after 6 weeks of treatment for both groups.
The demographic and clinical data were collected using a properly designed proforma. The data was then entered into version 25.0 of SPSS to be analyzed. Frequency and percentages were calculated for categorical variables while mean and standard deviation were calculated for continuous variables. The t-test was applied to assess the comparison of the dissimilarity in pain scores in the two groups with a p-value of ≤0.05 deemed significant. Effect modifiers were controlled by stratification.
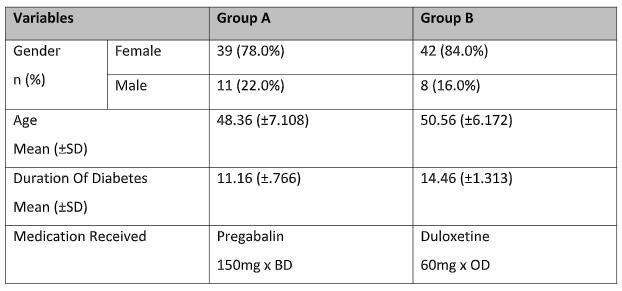
A total of 100 patients participated in the study and they were randomized equally in either group consisting of 50 patients each. All the patients completed the six-week trial and there were no dropouts. A majority of the participants were males 81 (81%). The mean age was calculated to be 49.46 years (SD± 6.64). The mean duration of diabetes in these patients was 12.81 years with a Standard deviation (SD ± 1.45). The baseline demographics of the two groups are given in Table 1.
Table 1: Baseline Demographics of the group A and B.
The mean pain score was calculated for the patients at baseline and then at the endpoint after 6 weeks of treatment. The mean score on the VAS at baseline for the sample was 6.83 (SD± .697) while the mean score for the participants after the clinical trial was 4.52 (SD± .588). The details of the mean scores on VAS for the two groups at baseline and 6 weeks are given in Table 2.
Table 2: Pain scores on the Visual Analog Scale (VAS) at baseline, week 3, and week 6 in both groups.
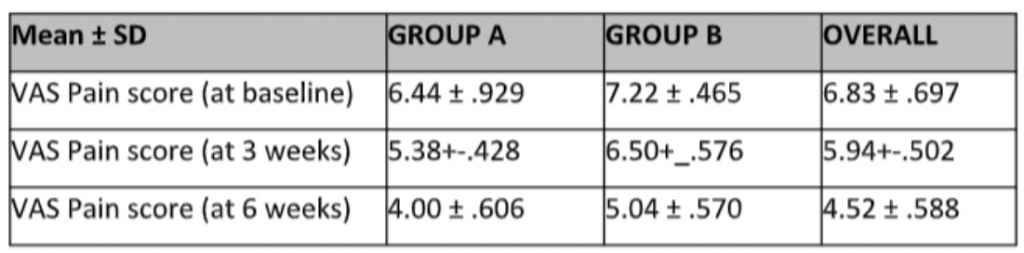
*t-test applied to compare the pain scores of two groups
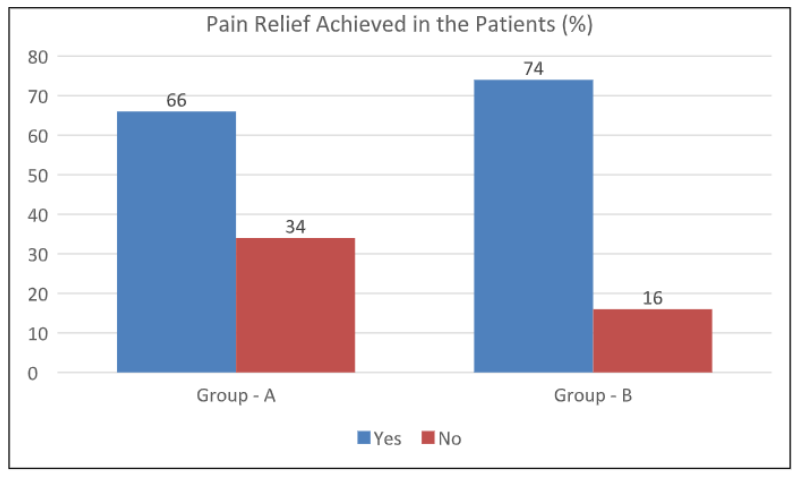
Pain relief was the primary outcome of the study. The primary outcome was defined as a drop in the VAS score of >50% at six weeks from that at the baseline. A total of 66% (n=33) in Group A and 74% (n=37) of the patients in Group B achieved pain relief. A comparison of the percentage of pain relief achieved between the two groups showed no statistical significance (p-value 0.383). Figure 1
Figure 1: Comparison of the percentage of pain relief in the two groups.
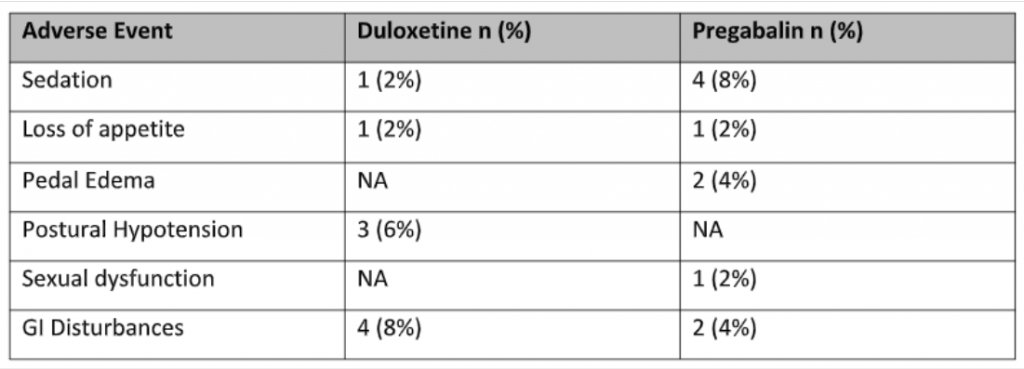
Few adverse effects were noted in the study though not statistically significant as shown in table 3. Some important to note was increased somnolence in the pregabalin group in 4 (8%) patients as compared to the duloxetine group, only 1 (2%) patient. 4 (8%) patients reported GI disturbances like vomiting and constipation in the Duloxetine group as compared to only 2 (4%) in the pregabalin group.
Table 3: Frequency of side effects experienced in both the study groups (n = 100)
One of the troubling and common complications of diabetes mellitus is the development of neuropathy13. It disrupts the quality of life of many diabetic patients and also burdens the health system. Newer medications are constantly undergoing trials for the relief of neuropathic pain in diabetics and many are now FDA-approved. Duloxetine which is an SSNRI. is one of these novel medications that is quite effective with a good safety profile14.
Diabetes mellitus is prevalent in adults aged 20 years or above in the US at around 12.9%. Moreover, the prevalence of impaired BSF (blood sugar fasting) is 25.7%, and impaired glucose tolerance is 13.8%. This predicts that more than 40% of adults aged >20 years have either diabetes mellitus or pre-diabetes, and this prevalence is on the rise15. Around 25% of diabetic patients suffer from symmetrical peripheral neuropathy and 7.5% to 24% of diabetics suffer from neuropathic pain16. On average 56% of type 1 diabetics and 41% of type 2 diabetics develop neuropathy in their lifetime, with diabetics having an incidence of 2% to develop symmetrical neuropathy annually17. The global epidemic of type 2 diabetes mellitus will eventually lead to a greater number of people being affected by peripheral neuropathic pain due to diabetes.18
Tricyclic anti-depressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have long been used primarily for painful peripheral neuropathy linked with diabetes19. They have been effective in some patients with milder pain according to many clinical trials20. The side effects of TCAs and SSRIs are troubling. They include gastrointestinal disturbances namely nausea, constipation, and diarrhea. They also include problems with sleep and sexual function making these medications difficult to take for longer duration21.
In contrast to TCAs and SSRIs, duloxetine hydrochloride and pregabalin are found to be more effective in pain relief in diabetic neuropathy with a better safety profile, making them more feasible to take for longer periods. Both drugs are approved by the FDA (Food & Drug Administration). Duloxetine is also effective in certain other disorders like depression, anxiety, and fibromyalgia22,23. In our study, the mean + standard deviation of the age of patients in the Pregabalin and Duloxetine groups were 48.36+7.10 and 50.56+6.17 respectively. In a similar study, the age of patients in the Pregabalin group was 55.44+9.7 and for Duloxetine was 58.48+8.8 respectively24.
In a study, it was found that the frequency and percentages of male and female patients in the Pregabalin group were 32(61%) and 20(38.4%) respectively. Whereas the frequency and percentages of male and female patients in the Duloxetine group were 27(54%) and 23(46%) respectively25. In our study, the frequency and percentages of male patients in both groups were 39 (78.0%) and 42 (84.0%), and the frequency and percentages of female patients were 11 (22.0%) and 08(16.0%) respectively25. Devi et al in their study explained the mean and standard deviation (SD) that was estimated for pain (VAS) at baseline. In their study, Pregabalin was found as 64.9+18.9 in terms of VAS score at baseline. For the Duloxetine group, the mean and standard deviation for the VAS score was 57.1+ 16.125. Similarly, in our study, the mean pain (VAS) in the Pregabalin and duloxetine groups at baseline was 6.44+0.92 and 7.22+0.46 respectively.
Some limitations of our study were a smaller sample size or group and a localized study area. Involving more people belonging to different geographical locations and social circles would make the study more superior.
In conclusion, data from our study reveals that both duloxetine and pregabalin are effective therapies for pain relief in painful diabetic neuropathy as evidenced by the VAS pain score in both groups A and B after 3 and 6 weeks of treatment duration. Treatment with duloxetine gave slightly better pain relief than pregabalin for this disabling complication of diabetes according to our study, though not statistically significant as also seen in previous studies. The adverse effects of both therapies were minimal.
I like to acknowledge the hospital staff and doctors for their immense contribution.
All the authors at this moment declare that there is no conflict of interest.
Ethical Approval was obtained from HBS General Hospital with reference number EC18/169,15thOCT 2022.
Informed consent was obtained from the patient before enrolling in the study.
HR: Major contribution in writing the manuscript, NZ: Major contribution in writing the manuscript, AZ: Major contribution in writing the manuscript, HS: Major contribution in writing the manuscript, MT: Data Collection and Analysis, AB: Formal Analysis and Editing.
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. doi: 10.1038/s41572-019-0092-1.
- Frier BM, Fisher M. Diabetes mellitus. In: Colledge NR, Walker BR, Ralston SH (Editors). David’s principles and practice of Medicine. 21sted.New Delhi: Elsevier Limited 2010; 796-834.
- Akhtar S, Hassan F, Saqlain SR, Ali A, Hussain S. The prevalence of peripheral neuropathy among the patients with diabetes in Pakistan: a systematic review and meta-analysis. Sci Rep. 2023;13(1):11744. doi: 10.1038/s41598-023-39037-1.
- Castelli G, Desai KM, Cantone RE. Peripheral Neuropathy: Evaluation and Differential Diagnosis. Am Fam Physician. 2020;102(12):732-739.
- Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care. 2009;3(2):136-143. doi: 10.1097/SPC.0b013e32832b7df5.
- Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456-2465. doi: 10.2337/dc12-1964.
- Wright A, Luedtke KE, Vandenberg C. Duloxetine in the treatment of chronic pain due to fibromyalgia and diabetic neuropathy. J Pain Res. 2010; 4:1-10. doi: 10.2147/JPR.S12866.
- Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care. 2011;34(4):818-822. doi: 10.2337/dc10-1793.
- NICE Clinical Guideline 96: Neuropathic Pain. The pharmacological management of neuropathic pain in adults in non-specialist settings.2010.
- Wu CS, Huang YJ, Ko YC, Lee CH. Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. Syst Rev. 2023;12(1):53. doi: 10.1186/s13643-023-02185-6.
- Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T, Shoji S. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011 Jan;28(1):109-116. doi: 10.1111/j.1464-5491.2010.03152.x.
- Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi: 10.1212/WNL.0b013e3182166ebe.
- Vinik A. The approach to the management of the patient with neuropathic pain. J Clin Endocrinol Metab. 2010;95(11):4802-4811. doi: 10.1210/jc.2010-0892.
- Armstrong DG, Chappell AS, Le TK, Kajdasz DK, Backonja M, D’Souza DN, Russell JM. Duloxetine for the management of diabetic peripheral neuropathic pain: evaluation of functional outcomes. Pain Med. 2007;8(5):410-418. doi: 10.1111/j.1526-4637.2007.00276.x.
- Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes. 2015;6(3):432-444. doi: 10.4239/wjd.v6.i3.432.
- Pritchett YL, McCarberg BH, Watkin JG, Robinson MJ. Duloxetine for the management of diabetic peripheral neuropathic pain: response profile. Pain Med. 2007;8(5):397-409. doi: 10.1111/j.1526-4637.2007.00305.x.
- Whitmyer VG, Dunner DL, Kornstein SG, Meyers AL, Mallinckrodt CH, Wohlreich MM, Gonzales JS, Greist JH. A comparison of initial duloxetine dosing strategies in patients with major depressive disorder. J Clin Psychiatry. 2007;68(12):1921-1930. doi: 10.4088/jcp.v68n1213.
- Skljarevski V, Zhang S, Iyengar S, D’Souza D, Alaka K, Chappell A, Wernicke J. Efficacy of Duloxetine in Patients with Chronic Pain Conditions. Curr Drug ther. 2011;6(4):296-303. doi: 10.2174/157488511798109592.
- Yang H, Sloan G, Ye Y, Wang S, Duan B, Tesfaye S, Gao L. New Perspective in Diabetic Neuropathy: From the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front Endocrinol (Lausanne). 2020; 10:929. doi: 10.3389/fendo.2019.00929.
- Shahid W, Kumar R, Shaikh A, Kumar S, Jameel R, Fareed S. Comparison of the Efficacy of Duloxetine and Pregabalin in Pain Relief Associated with Diabetic Neuropathy. Cureus. 2019;11(7):e5293. doi: 10.7759/cureus.5293.
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol. 2008; 8:29. doi: 10.1186/1471-2377-8-29.
- Wernicke JF, Wang F, Pritchett YL, Smith TR, Raskin J, D’Souza DN, Iyengar S, Chappell AS. An open-label 52-week clinical extension comparing duloxetine with routine care in patients with diabetic peripheral neuropathic pain. Pain Med. 2007;8(6):503-513. doi: 10.1111/j.1526-4637.2006.00258 x.
- Shaheen A, Alam SM, Ahmad A, Khan M. Clinical efficacy and tolerability of Gabapentinoids with current prescription patterns in patients with Neuropathic pain. Pak J Med Sci. 2019;35(6):1505-1510. doi: 10.12669/pjms.35.6.652.
- Devi P, Madhu K, Ganapathy B, Sarma G, John L, Kulkarni C. Evaluation of efficacy and safety of gabapentin, duloxetine, and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J Pharmacol. 2012;44(1):51-56. doi: 10.4103/0253-7613.91867.
- Tanenberg RJ, Irving GA, Risser RC, Ahl J, Robinson MJ, Skljarevski V, Malcolm SK. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc. 2011;86(7):615-626. doi: 10.4065/mcp.2010.0681.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
