By Samama Arshad1, Fatima Jehangir2, Murtaza Muzammil3, Tariq Adnan4
- Medilink Consultant Clinics, Karachi, Pakistan
- Community Health Sciences Department, Karachi Medical and Dental College, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD12-4/006
How to cite: Arshad S, Jehangir F, Muzammil M, Adnan T. Post COVID-19 Syndrome: Assessment of Short- and Long-Term Post-Recovery Manifestations Among Pakistani Community. Pak J Med Dent. 2023;12(4): 25-31. Doi: 10.36283/PJMD12-4/006
Background: The long-term effects of SARS-CoV-2 infection are becoming a significant cause of concern for society and healthcare systems. This study determined the variety of symptoms short and long-term after recovery from COVID-19 infection.
Methods: A cross-sectional study of 100 subjects followed for 2 years, all COVID-19 infection survivors (RT-PCR positive), 18 years and above were recruited. Participants were examined and screened with WHO post-COVID assessment criteria for post-acute (>2 weeks) and long-term (>4 weeks) symptoms that lingered on post-recovery of acute illness. Chi-square was used for the association with the outcome variable with p-value < 0.05 was significant.
Results: The mean age of the participants was 37.8+10.3 years with the majority 55% being men. There was complete resolution of symptoms in 86% whereas 14% had lingering symptoms post-COVID (p=0.549). The most common severe symptoms in the post-acute phase (after 2 weeks) in COVID recoveries were fatigue, myalgias followed by anosmia and ageusia. Persistent long-term (after 4 weeks) presentations in the COVID survivors were cardiac and neurological complications such as CAD (coronary artery disease) and Bell’s palsy followed by long-term fatigue/generalized weakness and dizziness. Mental health sequelae post covid, most subjects had insomnia (27%) and amnesia (27%) followed by anxiety (20%), depression (14%), and brain fogging (12%) (p-value 0.347).
Conclusion: The majority of COVID-19 survivors achieved complete symptom resolution, with only 14% experiencing lingering symptoms. These findings contribute to our understanding of the diverse and complex manifestations of COVID-19 recovery but highlight the need for further research to elucidate the underlying mechanisms.
Keywords: COVID-19, Post-Acute COVID-19 Syndrome, Recovery.
The COVID-19 pandemic has had a significant impact on global public health. Despite considerable efforts to control its spread, a substantial proportion of patients who have recovered from acute COVID-19 continue to experience a range of symptoms, often persisting for weeks or even months after the initial infection. Understanding these long-term effects is crucial for providing appropriate medical care and support to affected individuals. Many studies revealed that COVID‐19 has the potential to cause long‐term effects on nearly all systems including respiratory, cardiovascular, gastrointestinal, neurological, and psychiatric manifestations1.
A significant percentage of patients (87.5%) who had improved after acute infection continued to endure a diversity of symptoms such as fatigue, dyspnea, cough, myalgia, and headache2,3. Published data indicated that individuals who experienced mild illness or had no apparent symptoms may also suffer from long‐term symptoms. The terminology regarding ongoing symptoms after recovery and the resolution of the disease differ among studies and societies’ definitions as the Centers for Disease Control and Prevention (CDC) and The National Institute for Health and Care Excellence (NICE)4,5 continuing symptoms beyond 3 weeks from the disease onset were designated as post‐acute COVID‐19 and beyond 12 weeks as chronic COVID‐196.
The US CDC suggested separating the disease into different categories as acute COVID‐19 (the first 2 weeks from symptom start), post‐acute hyper inflammatory illness (between 2 and 4 weeks from symptoms start), and late period symptoms (more than 4 weeks from symptom start)7.
The involvement of the cardiovascular system in COVID-19 has been widely reported. Patients have shown signs of myocardial injury, arrhythmias, and blood clotting disorders during the acute phase. Emerging evidence suggests that some individuals may continue to suffer from these cardiac issues well into the post-acute and chronic phases of the disease. Gastrointestinal manifestations, such as diarrhea, nausea, and loss of appetite, have been observed in COVID-19 sufferers, and persistent gastrointestinal symptoms in recovered patients are also seen, suggesting potential long-term effects on the digestive system8.
Neurological complications of COVID-19, including anosmia (loss of smell), ageusia (loss of taste), headaches, and cognitive impairment, have been frequently reported. Moreover, psychiatric symptoms such as anxiety, depression, and post-traumatic stress disorder have been observed in COVID-19 survivors, potentially impacting their overall well-being and quality of life9.
The long-term effects of COVID-19 on multiple organ systems are increasingly recognized, affecting a significant proportion of recovered patients. Even individuals with mild or asymptomatic COVID-19 may not be exempt from experiencing ongoing symptoms. To better understand and manage these sequelae, further research and standardized definitions are required. Healthcare providers must remain vigilant in monitoring and supporting patients with post-acute and chronic COVID-19 to optimize their recovery and well-being. The study aimed to explore the long-term manifestations of COVID-19 on various organs in the Pakistani population so that we could link the underlying mechanisms of these long-term effects and develop effective therapeutic strategies to improve patient outcomes.
A cross-sectional study was done on n=100 COVID-19 infection survivors 18 years and above. Study duration was 2 years from Jan 2021 to December 2022. The inclusion criteria for acceptability into the study included all COVID-19 survivors who have consented to participate and are attending clinic services in the OPD and who were COVID-19 RT-PCR Positive. Exclusion criteria included those who refused to give consent. They were called for follow-up once every month and thorough history and examination were done by consultant family physicians to exclude any acute infection or uncontrolled status of any chronic disease. Also, new symptom appearing and lasting long-term was documented.
Participants were examined and screened with WHO post-COVID assessment criteria for post-acute (>2 weeks) and long-term (>4 weeks) symptoms that lingered on post-recovery of acute illness. The study was approved by the institutional review board with reference number, ERC code# 4780122SAFM. SPSS version 20 was used for statistical analysis. Mean and standard deviation were computed for numerical variables and frequencies and percentages for categorical variables. Chi-square was computed to see the association with the outcome variable. P-value < 0.05 was considered significant.
The mean age of the participants was 37.8+10.3 years. The majority (55%) were men and there were 45% women. The most common severe symptoms in the post-acute phase (after 2 weeks) in COVID recoverees were fatigue, myalgias followed by anosmia, and ageusia. Persistent long-term (after 4 weeks) presentations in the COVID survivors were cardiac and neurological complications such as CAD (coronary artery disease) and Bell’s palsy followed by long-term fatigue/ generalized weakness and dizziness. Table 1 demonstrates the demographic profile of the participants. Table 2 compares the symptoms occurring in patients during COVID-19 infection and how much of them lingered post 2 weeks of acute COVID-19 infection. When we looked at the mental health sequelae post covid, most subjects had insomnia and amnesia followed by anxiety, depression, and brain fogging but the results are statistically insignificant (p-value 0.347). The majority of patients (74%) were otherwise healthy having no comorbidities however 15% had 1 comorbid condition, 8% had 2 and only 1% had more than 3 conditions.
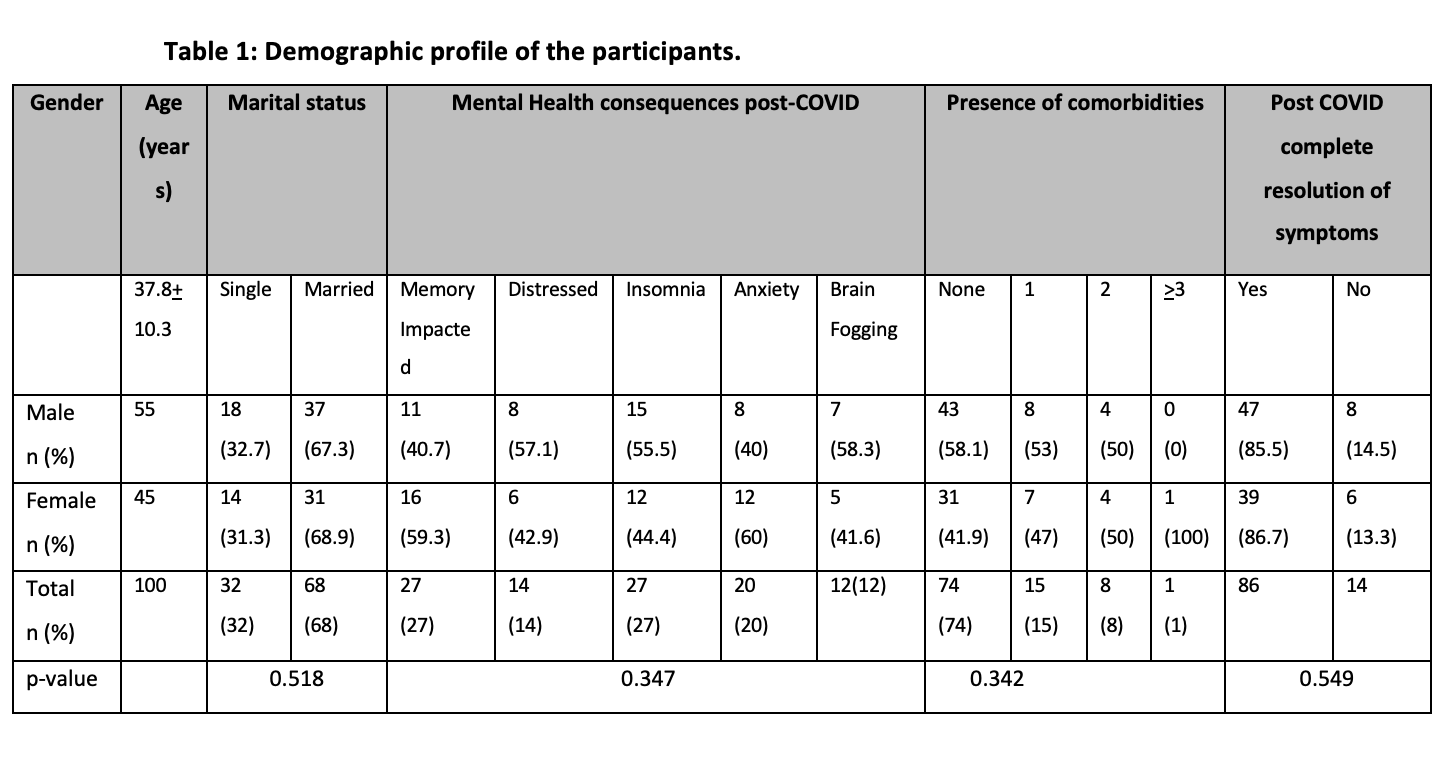
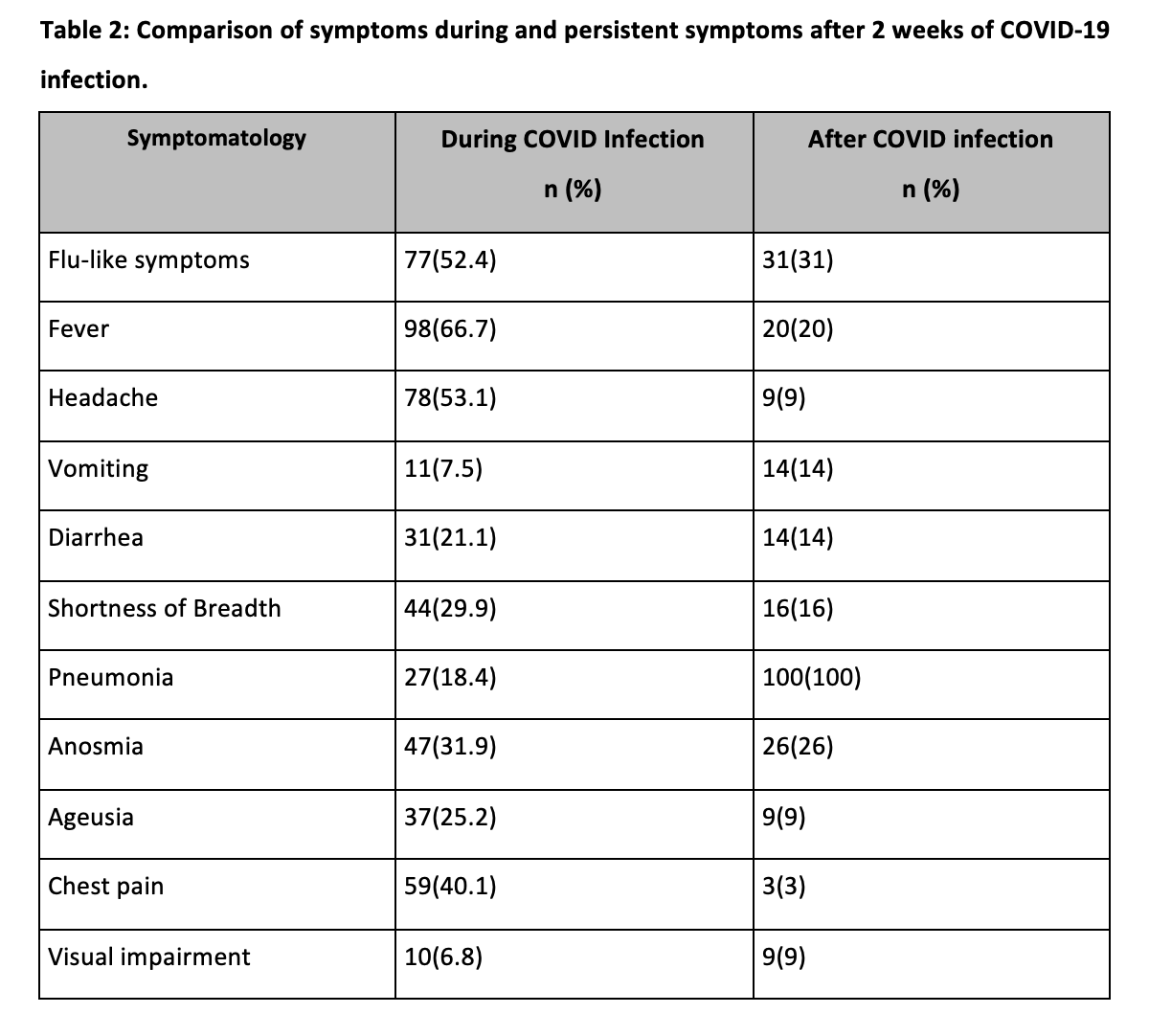
Figure 1 shows the short-term consequences after two weeks of COVID-19 infections, with fatigue, and myalgias being the most common.
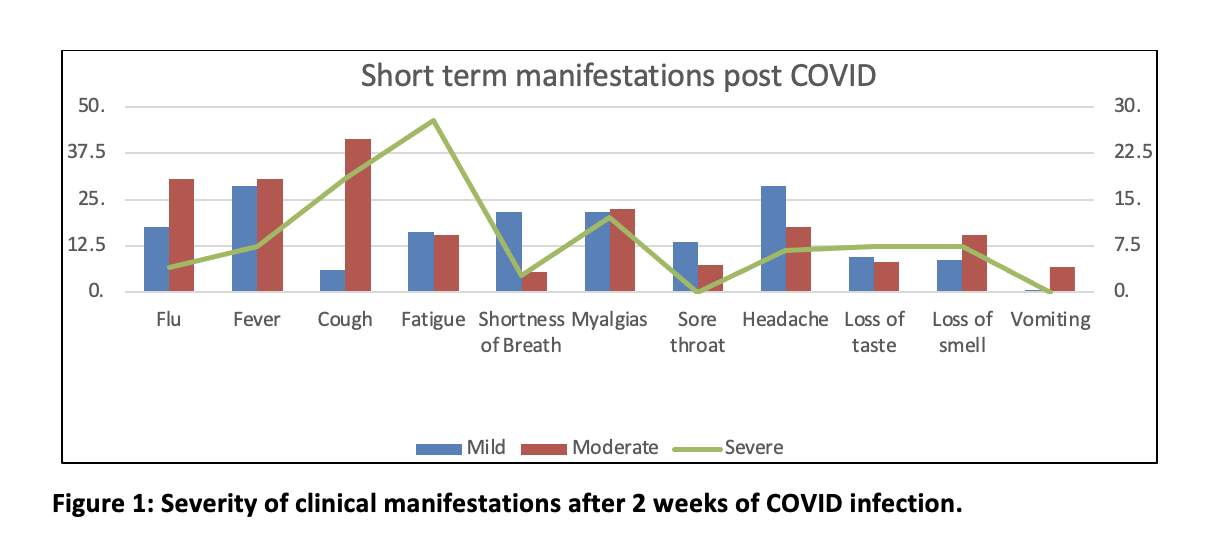
Figure 2 illustrates the various long-term i.e., beyond 4 weeks, complications post-infection, and long-term fatigue were seen the most followed by CAD, Bell’s Palsy, and dizziness. 14% of COVID survivors could not return to their baseline functionality and remained sick with long-lasting symptoms (p-value 0.549).
The severe acute respiratory syndrome called the Coronavirus 2 (SARS-COV- 2) infection has a wide range of presentations; from asymptomatic cases to acute respiratory syndrome, multi-organ failure, or residual symptoms in recovered patients called Long COVID or post-COVID syndrome which is a new medical challenge with unclear long-term sequelae. The data shows that 10-35% of patients treated in the community and 85% of patients requiring inpatient treatment develop post-COVID syndrome or Long COVID 8. Post-COVID syndrome is a pathological condition involving multiple organ systems leading to chronic medical, mental, and psychological complications which result in poor quality of life, increased patient frustration, and increased mortality risk 9.
The most common symptoms of Long COVID are extreme tiredness or fatigue, shortness of breath, dyspnea, loss of sense of smell and or taste, and muscle aches or Myalgia 10. The most commonly reported mental symptoms are memory and concentration deficits referred to as “Brain Fog”, insomnia, and anxiety. Other reported symptoms are chest pain or tightness, Heart palpitations, dizziness, pins and needles, joint pain, depression and anxiety, tinnitus, earache, feeling sick, diarrhea, stomach aches, loss of appetite, increased temperature, headache, sore throat, and rashes 11.
Similarly, Song et al reported a cough prevalence of 18% (95% CI, 12-24%, n=14) weeks after COVID-19 onset 12. Cohort data in Norway and the Faroe Islands found that about 10% of COVID-infected patients who were treated as outpatients were coughing up to 4 months after the onset of infection 13,14. It is important to understand the pathophysiology of the cough symptoms associated with COVID-19 infection to develop a treatment for COVID-19 infection. Song et al propose that the cough hypersensitivity is due to sensory vagal neuron invasion by SARS-CoV-2, causing neuro-inflammation in central and peripheral cough pathways 12.
In a study conducted by Huang Y et al., Lung function tests were conducted on a group of 57 patients after being diagnosed with COVID-19 infection. 53% of these patients showed decreases in lung diffusion capacity for carbon monoxide (DLCO), and 49% of these patients were found to have decreased strength in their respiratory muscles. In another study, abnormalities of lung function tests were found in a significant number of patients (14/55) recovering from inpatient treatment. Lung diffusion impairment was also found in another group of patients even in their six-month post-COVID follow-up visit 15. Our cohort did not show shortness of breath lasting after 2 weeks of infection. Perhaps it had resolved spontaneously as the infection improved, also the patients were younger and with low co-morbidities. Our data also reported severe Anosmia in 12.5 % of participants at 2 weeks’ post-infection and persisted beyond 4 weeks in a significant number of patients recovering from COVID-19 infection.
In a study conducted at the University of Pennsylvania, 98% of COVID-infected patients exhibited some altered sense of smell. Tara J. Wu et al concluded in their systemic review that up to 68% of patients who are infected with COVID-19 reported Anosmia within 2 weeks and persisted in a small number of patients beyond 2 weeks 16. Our study findings 2 weeks post-COVID correlate with the cough, shortness of breath, and Anosmia cluster of symptoms that occur post-COVID as most of our participant displayed a combination of cough, dyspnea, and anosmia as their predominant symptom. In a Multicenter study carried out by Cesar Fernandez et al, they followed up with telephone interviews of patients who recovered from COVID-19 after they were treated at three different public hospitals in Madrid, Spain. They interviewed 1,950 patients and found that only 367 (18.8%) patients were symptom-free. The prevalence of long-term cough, dyspnea, and fatigue was reported at 2.5%, 23.3%, and 61.2% respectively 17.
Our long-term data after 4 weeks post-COVID showed the most common complaints of fatigue, headache, impaired memory, and cardiac symptoms like chest pain, palpitation, and coronary artery disease. Researchers have suggested that the altered processing of sensory stimuli by the central nervous system and the peripheral pathways as causes of ongoing fatigue after COVID-19 infection 18. Interestingly a correlation between dyspnea, pain, and cough can be explained based on functional MRI studies of the brain showing Insular and Cingulate cortices as principal areas of the brain dealing with nociceptive processing of dyspnea, cough, and pain 19. Available scientific literature also shows a possible link between Long COVID and cardiac symptoms including chest pain. Cardiac Magnetic resonance imaging (MRI) of 12/26 (46%) of college athletes with confirmed COVID infection showed evidence of myocarditis or myocardial injury after 12 – 53 days from diagnosis 20. Another prospective observational cohort study of 100 post-COVID patients from University Hospital Frankfurt indicated that 78% of participants had abnormal cardiac MRI 2-3 months after acute COVID-19 infection. Of these 100 patients, 60% had evidence of myocardial inflammation 21. One other study claimed that 40% of post-COVID patients presented with Pericarditis or Myocarditis more than 70 days’ post-infection 22.
In this study, it was also noted a significant number of patients reported mental health problems such as cognitive impairment, memory issues, insomnia, brain fog, anxiety, and despair. A single-center, retrospective cohort study of adult patients was carried out by Karen Keijsers et al 23. They used the Hospital Anxiety and Depression Scale (HADA) to screen mental health in COVID-19 survivors. Their cohort included two hundred and eleven patients with a median age of 63 years. 33% of patients reported memory impairment and 28% had complaints of concentration problems. Further results showed anxiety and depression occurring among 7.8% and 7.1% respectively 23. In a similar study, Tehmina Nasserie et al reported depression and/or anxiety with a median frequency of 14.9% and 2.21%. They also reported impaired cognitive function at a median frequency of 28.3% 24. Data collected by Huang et al, Akter et al and Halpin et al reported combined occurrences of anxiety and depression symptoms in post-COVID patients at 21.1%, 21.6%, and 23.0% respectively 25,26.
To fight COVID-19 and its long-term consequences, it is important to do further research to explore and find answers to many questions. Some areas of further interest and research can be Racial differences in the occurrence and outcome of long COVID, what determines COVID-19 cluster presentations and chronic symptoms.
Post-COVID Syndrome or Long COVID is found as a multi-organ disease, presenting with a wide range of symptoms long after the acute infection subsides. This cluster of fatigue and pain points toward pathophysiological mechanisms involving the central nervous system rather than exclusively infectious processes. These post-symptoms affect patients’ quality of health and daily life. Therefore, it is important to identify post-COVID symptoms and their complications, as well as establish regular long-term follow-ups and use available interventions to treat symptoms and provide medical and social support as needed.
We are grateful to our patients and staff who participated in the study at Sikander Abad Clinic and Clifton Medical Clinic.
There was no conflict of interest among the authors.
The study was approved by the ethical review committee, ERC code# 4780122SAFM.
Written consent was taken from the parents of all participants.
SA and FJ were involved in conceiving the idea, benchwork, and writing the manuscript. FJ did the statistical analysis. MM, TA, and FJ worked on manuscript writing and proofreading
- Adly HM, AlJahdali IA, Garout MA, Khafagy AA, Saati AA, Saleh SAK. Correlation of COVID-19 Pandemic with Healthcare System Response and Prevention Measures in Saudi Arabia. Int J Environ Res Public Health. 2020;17(18):6666. doi: 10.3390/ijerph17186666.
- Sivan M, Taylor S. NICE guideline on long COVID. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938.
- Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603.
- Datta SD, Talwar A, Lee JT. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications. JAMA. 2020;324(22):2251–2252. doi:10.1001/jama.2020.22717
- Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int J Environ Res Public Health. 2021;18(5):2621. doi: 10.3390/ijerph18052621.
- Halpin S, O’Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242-1243. doi: 10.1002/jmv.26587.
- Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, Pavkov ME, Rolka DB, Wiltz JL, Bull-Otterson L, Gray S, Boehmer TK, Gundlapalli AV, Siegel DA, Kompaniyets L, Goodman AB, Mahon BE, Tauxe RV, Remley K, Saydah S. Risk for Newly Diagnosed Diabetes >30 Days After SARS-CoV-2 Infection Among Persons Aged <18 Years – United States, March 1, 2020-June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):59-65. doi: 10.15585/mmwr.mm7102e2.
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch Med Res. 2021;52(6):575-581. doi: 10.1016/j.arcmed.2021.03.010.
- Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, Reid TR. A Review of Persistent Post-COVID Syndrome (PPCS). Clin Rev Allergy Immunol. 2023;64(1):66-74. doi: 10.1007/s12016-021-08848-3.
- Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, Haroon S, Price G, Davies EH, Nirantharakumar K, Sapey E, Calvert MJ; TLC Study Group. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428-442. doi: 10.1177/01410768211032850.
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-631. doi: 10.1038/s41591-021-01292-y.
- Song WJ, Hui CKM, Hull JH, Birring SS, McGarvey L, Mazzone SB, Chung KF. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9(5):533-544. doi: 10.1016/S2213-2600(21)00125-9.
- Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76(4):405-407. doi: 10.1136/thoraxjnl-2020-216377.
- Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, Á Steig B, Gaini S, Strøm M, Weihe P. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin Infect Dis. 2021;73(11):e4058-e4063. doi: 10.1093/cid/ciaa1792.
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8.
- Wu TJ, Yu AC, Lee JT. Management of post-COVID-19 olfactory dysfunction. Curr Treat Options Allergy. 2022;9(1):1-18. doi: 10.1007/s40521-021-00297-9.
- Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V, Torres-Macho J. Prevalence of Post-COVID-19 Cough One Year After SARS-CoV-2 Infection: A Multicenter Study. Lung. 2021;199(3):249-253. doi: 10.1007/s00408-021-00450-w.
- Rivera C, Mateo. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics (Basel, Switzerland). 2019;9(3):91. doi:10.3390/diagnostics9030091.
- Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, Million M, Raoult D, Cammilleri S, Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823-2833. doi: 10.1007/s00259-021-05215-4.
- Rajpal S, Tong MS, Borchers J, et al. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021;6(1):116–118. doi:10.1001/jamacardio.2020.4916
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. doi:10.1001/jamacardio.2020.3557
- Eiros, R.Pericarditis and myocarditis long after SARS-CoV-2 infection: a cross-sectional descriptive study in health-care workers. medRxiv. 2020. doi:10.1101/2020.08.09.20171104.
- Keijsers K, Broeders M, Baptista Lopes V, Klinkert A, van Baar J, Nahar-van Venrooij L, Kerckhoffs A. Memory impairment and concentration problems in COVID-19 survivors 8 weeks after non-ICU hospitalization: A retrospective cohort study. J Med Virol. 2022;94(9):4512-4517. doi: 10.1002/jmv.27831.
- Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495-503. doi: 10.1001/jama.2010.851.
- Akter F, Mannan A, Mehedi HMH, Rob MA, Ahmed S, Salauddin A, Hossain MS, Hasan MM. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab Syndr. 2020;14(6):2031-2038. doi: 10.1016/j.dsx.2020.10.016.
- Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T, O’Connor RJ, Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93(2):1013-1022. doi: 10.1002/jmv.26368.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
