By Akhtar Ali1, Nimra Khalid2, Syed Hasan Danish3, Syed Wajid Shah4
- Pharmacology Department, Ziauddin Medical College, Karachi, Pakistan.
- Healthcare Management Department, Ziauddin Medical College, Karachi, Pakistan.
- Community Health Sciences Department, Ziauddin Medical College, Karachi, Pakistan.
- Pharmacy Practice Department, Ziauddin University, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD12-4/015
How to cite: Ali A, Khalid N, Danish SH, Shah SW. No Action Today, No Cure Tomorrow – A Review of Agricultural Factors Predisposing Humans at The Risk of Antimicrobial Resistance and Its Preventive Strategies. Pak J Med Dent. 2023;12(4): 80-85. doi:10.36283/PJMD12-4/015
One of the most significant public health issues today is AMR (AMR), which poses significant difficulties in controlling and treating infectious diseases. It is believed that the abuse and overuse of numerous antibacterial agents in the healthcare and agriculture industries led to the emergence of antimicrobial resistance. Even though irrational prescriptions have been blamed for the rise in resistance among microbes to various antimicrobial drugs, a variety of environmental factors have also been linked to the development of multidrug-resistant (MDR) organisms. Several studies have stressed the significance of using a one-health approach, or a holistic strategy, to combat antibiotic resistance that takes into account people, animals, and the environment. Models describing how resistance starts and spreads could be created with a better understanding of the environmental factors that encourage its development. Yet, preventing the establishment and spread of mobile resistance elements is challenging since it is unclear how and when the environment favors resistance growth. Hence the current review focuses on the agricultural factors predisposing humans to the risk of AMR and preventive strategies.
Keywords: Antimicrobial Drug Resistance, Agriculture, Prevention and Control.
Antimicrobial resistance (AMR) is now being considered among significant public health concerns, providing substantial barriers to infectious disease treatment and prevention. Despite various efforts being made in current times to address this issue, global AMR trends display no sign of abating1. AMR is assumed to have evolved as a result of the inappropriate and overuse of numerous antibacterial drugs in both the medical and agricultural sectors. AMR is additionally impacted by the process of bacterial evolution, bacterial mutation, and the horizontal gene transfer of resistant genes2. Though the increase in resistance among microorganisms towards various antimicrobial agents has been attributed to irrational prescriptions, however, various environmental factors are responsible for the development of multi-drug resistant (MDR) organisms 3. Several researchers have emphasized the importance of taking a holistic approach to tackle antibiotic resistance that considers animals, humans, and the environment—a so-called one-health approach. An increased understanding of the environmental elements that facilitate the development of resistance could lead to the generation of models for how resistance originates and spreads. However, mitigating the creation and spread of mobile resistance elements is difficult due to a lack of understanding of how and under what conditions the environment favors resistance development. Furthermore, irrational and unmonitored use of antibiotics in various fields of agriculture (Plants, Livestock, Crops) facilitates in development of AMR4, 5. Therefore, the current review focuses on the agricultural factors that are responsible for predisposing humans to the risk of AMR and strategies to combat it 6, 7.
This review study discusses the agricultural factors predisposing humans at the risk of antimicrobial resistance and strategies to combat it. The related literature was explored by using public search engines and electronic databases like MEDLINE, PUBMED, Google Search, and Google Scholar. The proper keywords for the topic-specific search were “Antimicrobial resistance, Agricultural factors, Improvements in prevention, ways to combat antimicrobial resistance established as a result of agricultural goods, etc.
AMR has become a serious public health risk around the world, with 10 million fatalities anticipated by 2050. AMR emerges when antimicrobial treatments are ineffective against various pathogens such as “viruses, bacteria, fungi, and parasites”, allowing the microorganism to survive within the host. AMR has been referred to as the “Silent Pandemic” and AMR is a concern that requires immediate attention and should be controlled more effectively rather than being seen as an issue that might arise in the future8. One of the primary contributors to the existing problem can be the consequences of antibiotic abuse or irresponsible usage in a range of situations, mostly clinical treatment, but also agricultural use, animal healthcare, and the food system9. Several recent researchers have found that the use of antimicrobials in food systems and agriculture may have a substantial influence on AMR. According to reports, 70% of antibiotics used for treating humans are also being used in veterinary medicine in the United States. Recognizing the broad use of antibiotics in global agriculture, several organizations, notably the World Health Organization (WHO), the European Union (EU), and the United Nations (UN) have taken initiatives to minimize and limit antimicrobial use in animals. Furthermore, legal restrictions on the use of various antibiotics in agri-food systems for growth promotion have been imposed, along with initiatives regarding antimicrobial stewardship programs in the treatment of food animals and small companies. However, such limitations may be hard to apply in emerging countries where demand for food animals continues to climb year after year. Hence, the review focuses on the agricultural factors that are responsible for predisposing humans to the risk of AMR and strategies to prevent it8, 10, 11.
Mechanisms of Development of Resistance in Bacteria
To evaluate the environmental factors that are causing the increase in antimicrobial resistance, it is important to understand the mechanisms that are responsible for the resistance development in bacteria 12. These mechanisms can be categorized as, (i) Intrinsic resistance, where the resistance is because of the bacterium’s inherent features such as enzyme (penicillinase) production by gram-positive organisms and the presence of glycopeptide layer in bacterial cell envelope’s outer membrane that prevents the entry of drugs inside the organism. (ii) Acquired resistance i.e., an earlier sensitive bacterium becomes resistant through the acquisition of new genes by gene transfer horizontally or through mutation, and (iii) Adaptive resistance i.e., antibiotic resistance triggered by a specific environmental stimulus. It subsides once the trigger is removed 7, 13, 14.
There are three approaches used by bacteria to acquire genetic material, (a) Transformation: This is a type of genetic recombination in which free DNA pieces from a dead bacterium are incorporated into the chromosome of a recipient bacterium. Only a few microorganisms can naturally change. (b) Transduction, A bacteriophage transfers genetic material from a donor to a recipient bacterium and (c) Conjugation, horizontal gene transfer is most likely accomplished using this technique. It includes the direct physical genetic material transmission from one cell of bacteria to another. A plasmid is transferred from the donor cell to the receiving cell through a sex pilus produced by the two bacterial cells. Multiple resistance genes are often located on a single plasmid, allowing for multidrug resistance to be delivered in a single conjugation event13, 15-17.
Agricultural Factors
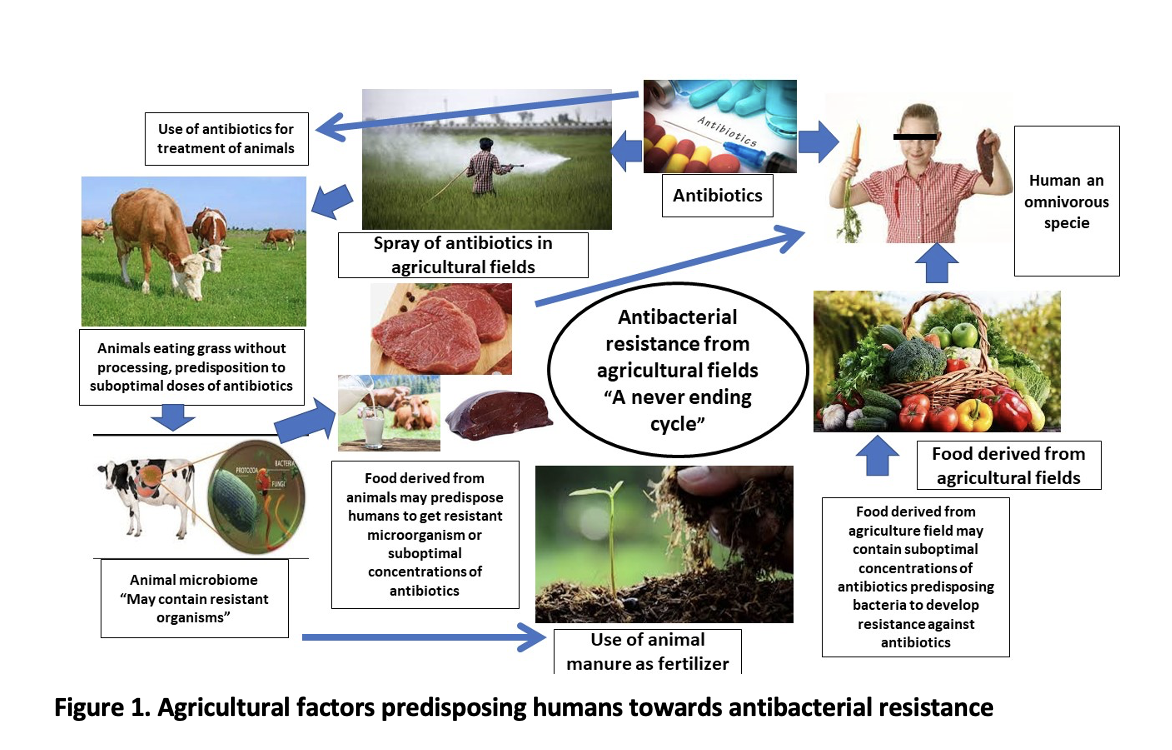
Soil has been considered to be a rich source of antibiotics and antibiotic-resistant genes due to natural and anthropogenic activities. Anthropogenic pressure may be caused by antibiotics used in healthcare or livestock that are discharged into the marine environment via wastewater treatment plants. Current water waste treatment plant systems only partially remove antibiotics, as well as other organic contaminants hence increasing the antibiotic concentration in the agricultural fields and providing exposure of different antibiotics to organisms that leads to the initiation of the resistance generation process. We found evidence that antibiotic-resistant genes can reach edible parts of commercial crops, and their concentrations and presence are affected by agricultural practices. Animal manure application has become a significant environmental concern 21. According to one study, when compared to mineral fertilizer, the usage of commercial organic fertilizer and composted manure significantly increased the abundance of antibiotic-resistant genes and pathogens, particularly in surface soil and pakchoi roots. The results of this study highlighted the vertical migration of antibiotic-resistant genes and pathogens in vegetables and soil and indicated that how the mutant genes or pathogens are transferred from agricultural fields to human hosts 22.
The antibiotics used in agricultural fields and grass or food used by animals as their chow are also thought to be a connective link between the transfer of resistant genes from agricultural fields to human hosts 23. The animals play a crucial role in this cycle. The grass or food used as their chow contain bacterial residue and also antibacterial compounds, the presence of both of these in animal-derived products (kidney, muscles, fat, liver, egg, and milk) leads to the transfer of resistant genes or organisms to human host as well as antibacterial residue in animal products also exposes the bacteria to suboptimal concentrations of antibiotics that further increases the resistance development risk 24, 25.
Understanding the cycle of transfer of resistant microorganisms and antibacterial residue at suboptimal doses from agricultural fields to humans can help in establishing policies to overcome the current scenario and also can be used to plan interventions to break this cycle that may help in reducing the burden or resistant organisms as well as antibiotic resistance that is posing a serious risk to society and if not intervened at right time this will lead us to era before 1928 when we were not having antibiotics to treat the infections 26-28.
Alternative Strategies to Encounter Antibiotic Resistance in the Agricultural Sector
As discussed earlier, the imprudent and widespread use of antibiotics in agriculture to boost crop output and in animals as growth promoters has resulted in the emergence of AMR to a large extent29. Keeping in view the extent of this major public health concern, numerous programs have been initiated and implemented worldwide by various government and public health specialists. Their recommended options include setup up of nationwide target for reduction in antibiotic use, good health practices, tracking down antimicrobial usage, tracking veterinarians and other healthcare professional who prescribe antibiotics, reliable diagnostics, and managing practices to reduce the spread 29-31.
Along with that, various strategies have been outlined to achieve advancements in the field of biotechnology which includes the use of carbohydrate-modified compounds, antimicrobial peptides, combination therapies, or the use of non-antimicrobials to develop new antibiotics with the potential to solve the global AMR problem32, 33.
Farming Practices
Carefully controlled extensive farming techniques using a slight amount of chemical substances that are good for animal health whilst preventing the occurrence of infectious diseases and lessening antibiotic usage should be encouraged instead of ancient farming practices 34. Organic farming practices have been deemed as an appropriate way to satisfy global food requirements while minimizing the excessive use of antimicrobial agents; nevertheless, further research is required to determine the features associated with such practices and boost production. 35. In 1986, Sweden implemented a complete ban on growth promotors in their food production industry and it is one of the World’s references to restrict the use of antibiotics in the food chain through well-regulated and adequate farming 32.
Immunization in Livestock
Due to increased global food requirements Farmers’ reliance on antibiotics or growth promoters to generate significant amounts of animal protein at a low cost has grown. However, evidence suggests that enhancing animal health and well-being can minimize antibiotic reliance without affecting cost or production. Utilization of vaccines is highly endorsed in livestock to prevent them from infections which eventually leads to a reduction in antibiotic consumption. To protect animal health, mass vaccination programs should be initiated, the higher the usage of vaccines, the lower the incidence of infections37, 38.
Ban on Unrestricted Antibiotic Use and Legislative Regulation
The unrestricted usage of antibiotics should be banned and only be allowed when it’s under a veterinary prescription. When classes of antibiotics permitted to prevent infectious disease in humans as well as animals are employed, substantial veterinary control should be emphasized 39. It is also essential to create ways for justifying antibiotic use, as well as indicators to track success and guidelines for taking veterinary medicines. However, the global burden of antibiotic resistance has outpaced the rate of new antibiotic development for clinical use, resulting in a drastic rethinking of antibiotic use 40. According to WHO, all LMICs should identify their annual consumption of antibiotics in animal food and prohibit the use of “critically, high, and important antimicrobials in human medicine” as well as those that are not currently approved for veterinary use. Achieving stability between minimized antibiotic use in the farming sector and fulfilling the unprecedented global food demand is very challenging as well and defining target goals to limit the use of antibiotics in the food chain is also a crucial step. It is therefore crucial that every country must involve all the stakeholders from different sectors to set realistic and achievable goals to reduce this burden 41-43.
Integrated Surveillance and Monitoring Systems
The determination of resistance load from diverse ecological niches in the food chain (farm, market, and human consumption) is a critical step in addressing this worldwide concern. A monitoring system for the integrated food chain is obligatory to develop. Therefore, to conduct sustainable epidemiological studies from farm to fork complying with one health approach, and reporting active, passive, and different outbreak data sources are important to fill these information gaps. Furthermore, to evaluate and monitor the impact of public awareness campaigns on antibiotic use and its adverse consequences on human health should be initiated 44, 45. The information that will be generated through such surveillance systems will eventually help the decision-makers and authorized people to make evidence-based decisions and effectively allocate the resources to prevent and reduce food chain-associated ABR46.
To cope with the situation, in November 2012, Pakistan formed its national body, the “Division of Pharmacy Services of the Drug Authority of Pakistan (DRAP)”, but the laws drafted have not yet been properly followed. Similarly, Pakistan has enacted a policy restricting the use of antibiotics in animal husbandry; however, the regulation has yet to be strictly enforced. Further to this, As part of its commitment to the global action plan to combat AMR, Pakistan has recently produced its national strategy framework for AMR containment, to convert it into an AMR National Action Plan (NAP) using the “One-Health” approach however, till date no outcome of any policy has been reported in this regard47.
The irrational use of antibiotics in agriculture has contributed to the development of MDR organisms. Hence, antibiotics usage in agriculture has a link to causing life-threatening infections in humans that are difficult to treat. Therefore, the usage of antibiotics in agriculture should be rationalized and policies for monitoring evaluation should be developed to precheck the agricultural products before distribution in the markets. Furthermore, awareness among the farmers regarding the use of antibacterial products should be promoted.
Author AA conceptualized the study, and formatted the draft facilitated in writing and literature search. Author NK and SWS managed the literature search and facilitated in writing of discussion and referencing. Author SHD critically reviewed the article and facilitated in finalization of the manuscript.
- Christaki E, Marcou M, Tofarides AJJome. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. 2020;88:26-40. doi: 10.1007/s00239-019-09914-3.
- Dadgostar PJI, resistance d. Antimicrobial resistance: implications and costs. 2019;12:3903. doi: 10.2147/IDR.S234610.
- Dhingra S, Rahman NAA, Peile E, Rahman M, Sartelli M, Hassali MA, et al. Microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. 2020;8:531. doi: 10.3389/fpubh.2020.535668.
- Hosain MZ, Kabir SL, Kamal MMJVW. Antimicrobial uses for livestock production in developing countries. 2021;14(1):210. doi: 10.14202/vetworld.2021.210-221.
- Chandra P, Mk U, Ke V, Mukhopadhyay C, U DA, V RJEOoDS. Antimicrobial resistance and the post-antibiotic era: better late than never effort. 2021;20(11):1375-90. DOI: 10.1080/14740338.2021.1928633
- Bengtsson-Palme J, Kristiansson E, Larsson DJJFmr. Environmental factors influencing the development and spread of antibiotic resistance. 2018;42(1):fux053. DOI: 10.1093/femsre/fux053
- Abushaheen MA, Fatani AJ, Alosaimi M, Mansy W, George M, Acharya S, et al. Antimicrobial resistance, mechanisms and its clinical significance. 2020;66(6):100971. DOI: 10.1016/j.disamonth.2020.100971
- Tang KWK, Millar BC, Moore JEJBJoBS. Antimicrobial Resistance (AMR). 2023;80:11387. DOI: 10.3389/bjbs.2023.11387
- Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, et al. Global trends in antimicrobial resistance in animals in low-and middle-income countries. 2019;365(6459):eaaw1944. DOI: 10.1126/science.aaw1944
- Pokharel S, Shrestha P, Adhikari BJAR, Control I. Antimicrobial use in food animals and human health: time to implement ‘One Health’approach. 2020;9:1-5. doi: 10.1186/s13756-020-00847-x
- McKernan C, Benson T, Farrell S, Dean MJJ-ar. Antimicrobial use in agriculture: Critical review of the factors influencing behaviour. 2021;3(4):dlab178. DOI: 10.1093/jacamr/dlab178
- Reygaert WCJAm. An overview of the antimicrobial resistance mechanisms of bacteria. 2018;4(3):482. DOI: 10.3934/microbiol.2018.3.482
- Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. 2016;387(10014):176-187.
- Lee J-HJJoM. Perspectives towards antibiotic resistance: from molecules to population. Springer; 2019. p. 181-184. DOI: 10.1016/S0140-6736(15)00473-0
- Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016 Apr;4(2):10.1128/microbiolspec.VMBF-0016-2015. DOI: 10.1128/microbiolspec.VMBF-0016-2015
- Christaki E, Marcou M, Tofarides AJJome. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. 2020;88(1):26-40. DOI: 10.1007/s00239-019-09914-3
- Vidovic N, Vidovic SJA. Antimicrobial resistance and food animals: Influence of livestock environment on the emergence and dissemination of antimicrobial resistance. 2020;9(2):52. DOI: 10.3390/antibiotics9020052
- Cerqueira F, Matamoros V, Bayona JM, Berendonk TU, Elsinga G, Hornstra LM, et al. Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis. 2019;177:108608. DOI: 10.1016/j.envres.2019.108608
- Olaimat AN, Al‐Holy MA, Shahbaz HM, Al‐Nabulsi AA, Abu Ghoush MH, Osaili TM, et al. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: a comprehensive review. 2018;17(5):1277-1292. DOI: 10.1111/1541-4337.12387
- Manaia CM, Rocha J, Scaccia N, Marano R, Radu E, Biancullo F, et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. 2018;115:312-234. DOI: 10.1016/j.envint.2018.03.044
- Zhou X, Wang J, Lu C, Liao Q, Gudda FO, Ling WJC. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. 2020;255:127006.
- Li H, Zheng X, Tan L, Shao Z, Cao H, Xu YJER. The vertical migration of antibiotic-resistant genes and pathogens in soil and vegetables after the application of different fertilizers. 2022;203:111884.
- Hayek MN. The infectious disease trap of animal agriculture. Sci Adv. 2022 Nov 4;8(44):eadd6681. doi: 10.1126/sciadv.add6681.
- Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules. 2018 Mar 30;23(4):795. doi: 10.3390/molecules23040795.
- Ma F, Xu S, Tang Z, Li Z, Zhang LJB, Health. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. 2021;3(1):32-38.
- Tyrrell C, Burgess CM, Brennan FP, Walsh F. Antibiotic resistance in grass and soil. Biochem Soc Trans. 2019 Feb 28;47(1):477-486. doi: 10.1042/BST20180552.
- Cania M., Manageiro V., Abriouel H., Moran-Gilad J., Franz C. M. A. P. (2018). Antibiotic resistance in foodborne bacteria. Trends Food Sci Technol. 84, 41–44. doi: 10.1016/j.tifs.2018.08.001
- Santacroce L, Bottalico L, Topi S, Castellaneta F, Charitos IA. The “Scourge of the Renaissance”. A Short Review About Treponema pallidum infection. Endocr Metab Immune Disord Drug Targets. 2020;20(3):335-343. doi: 10.2174/1871530319666191009144217.
- Aarestrup FM, Jensen VF, Emborg HD, Jacobsen E, Wegener HC. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am J Vet Res. 2010 Jul;71(7):726-33. doi: 10.2460/ajvr.71.7.726.
- Fereiduni E, Ghasemi A, Elbestawi MJM. Characterization of composite powder feedstock from powder bed fusion additive manufacturing perspective. 2019;12(22):3673. DOI: 10.3390/ma12223673
- Poupaud M, Putthana V, Patriarchi A, Caro D, Agunos A, Tansakul N, et al. Understanding the veterinary antibiotics supply chain to address antimicrobial resistance in Lao PDR: Roles and interactions of involved stakeholders. 2021;220:105943. DOI: 10.1016/j.actatropica.2021.105943
- ESCAP U. Climate change and population ageing in Asia-Pacific region: status, challenges and opportunities. 2022.
- Sinha R, Shukla PJP, letters p. Antimicrobial peptides: recent insights on biotechnological interventions and future perspectives. 2019;26(2):79-87. Sinha R, Shukla PJP, letters p. Antimicrobial peptides: recent insights on biotechnological interventions and future perspectives. 2019;26(2):79-87.
- Fischer K, Sjöström K, Stiernström A, Emanuelson UJJods. Dairy farmers’ perspectives on antibiotic use: A qualitative study. 2019;102(3):2724-2737. DOI: 10.3168/jds.2018-15015
- Price L, Stegger M, Hasman H, Aziz M, Larsen J, Andersen P, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 3: e00305-11. 2012. DOI: 10.1128/mBio.00305-11
- Tiseo K, Huber L, Gilbert M, Robinson TP, Van Boeckel TPJA. Global trends in antimicrobial use in food animals from 2017 to 2030. 2020;9(12):918. DOI: 10.3390/antibiotics9120918
- Woolhouse M, Ward M, Van Bunnik B, Farrar JJPTotRSBBS. Antimicrobial resistance in humans, livestock and the wider environment. 2015;370(1670):20140083. DOI: 10.1098/rstb.2014.0083
- McEwen SA, Collignon PJJMs. Antimicrobial resistance: a one health perspective. 2018;6(2):6.2. 10. DOI: 10.1128/microbiolspec.ARBA-0009-2017
- Aidara-Kane A, Angulo FJ, Conly JM, Minato Y, Silbergeld EK, McEwen SA, et al. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. 2018;7(1):1-8. DOI: 10.1186/s13756-017-0294-9
- Neethirajan S, Kemp BJS, Research B-S. Digital livestock farming. 2021;32:100408.
- Manage PM. Heavy use of antibiotics in aquaculture: Emerging human and animal health problems – A review. Sri Lanka Journal of Aquatic Sciences. 2018;23(1):13-27. DOI: https://doi.org/10.4038/sljas.v23i1.7543
- Clifford K, Desai D, Prazeres da Costa C, Meyer H, Klohe K, Winkler AS, Rahman T, Islam T, Zaman MH. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bull World Health Organ. 2018 Sep 1;96(9):662-664. doi: 10.2471/BLT.18.209585.
- Hernando-Amado S, Coque TM, Baquero F, Martínez JLJNm. Defining and combating antibiotic resistance from One Health and Global Health perspectives. 2019;4(9):1432-1442. DOI: 10.1038/s41564-019-0503-9
- Klerkx L, Jakku E, Labarthe PJN-Wjols. A review of social science on digital agriculture, smart farming and agriculture 4.0: New contributions and a future research agenda. 2019;90:100315. DOI: 10.1016/j.njas.2019.100315
- Groher T, Heitkämper K, Umstätter CJA. Digital technology adoption in livestock production with a special focus on ruminant farming. 2020;14(11):2404-2413. DOI: 10.1017/S1751731120001391
- Acar J, Moulin GJRST. Integrating animal health surveillance and food safety: the issue of antimicrobial resistance. 2013;32(2):383-392. DOI: 10.20506/rst.32.2.2230
- Ur Rahman S, Mohsin MJPVJ. The under reported issue of antibiotic-resistance in food-producing animals in Pakistan. 2019;1:1-16. DOI: 10.29261/pakvetj/2019.037
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
