By Depi Praharani1, Rina Sutjiati2, Sulistiyani3, Atik Kurniawati4, Dessy Rachmawati5, Dwi Prijatmoko2, Rudy Joelijanto2, Shierin Velly Fiolita6, Firda Qurrotul Aini Rasyid6, Lilis Nurhalifah6, Syafika Nuring Fadiyah6, Millenieo Martin6
- Periodontics Department, Faculty of Dentistry, University of Jember, Jember, Indonesia.
- Orthodontics Department, Faculty of Dentistry, University of Jember, Jember, Indonesia.
- Pedodontics Department, Faculty of Dentistry, University of Jember, Jember, Indonesia.
- Oral Biology Department, Faculty of Dentistry, University of Jember, Jember, Indonesia.
- Biomedical Science Department, Faculty of Dentistry, University of Jember, Jember, Indonesia.
- General Dentistry Department, Faculty of Dentistry, University of Jember, Jember, Indonesia.
DOI: https://doi.org/10.36283/PJMD12-4/003
How to cite: Praharami D, Sutjiati R, Sulistiyani, Kurniawati A, Rachmawati D, Prijatmoko D, Joelijanto R, Fiolita SV, Rasyid FQA, Nurhalifah L, Fadiyah SN, Martin M. Expression of TNF-α and TGF-β after the Administration of Cacao Bean Extract on Alveolar Bone. 2023;12(4): 5-10. Doi: 10.36283/PJMD12-4/003
Background: Teeth that are given orthodontic forces can relapse because of excessive resorption on the pressure side. Cocoa bean extract enhances bone apposition during bone remodeling by increasing osteoblast proliferation. This research aimed to ascertain molecular mechanisms of alveolar bone during orthodontic tooth movement applied with cacao bean extract (Theobroma cacao L.) through TNF-α and TGF-β, expressions.
Methods: This study utilized an experimental laboratory posttest-only control group design. The NiTi closed coil spring was braced between the right upper first molar and the upper incisor, a strength of 10gf was estimated utilizing a tension gauge to mesially move the upper molars. The total 36 were divided into six groups, Wistar rats were beheaded on days 7 and 14 of treatment. Immunohistochemical staining was utilized to show TNF-α and TGF-β expression. Results were assessed using the one-way ANOVA analysis.
Results: The immunohistochemistry findings in osteoclast cells that demonstrate positive results for TGF-β and TNF-α, expression by immunohistochemical staining calculated for each group (control, positive control, treatment). The treatment group (Q14) showed reduced expression (2±0.957) of TNF-α in osteoclast cells. TGF-β, on the other hand, was found with diminished expression in osteoblast cells C-7 and C-14 (3.75±0.816, 3.25±0.957) and increases in the treatment group (Q14) (11.25±0.957). significantly (p=0.000).
Conclusion: The administration of cacao bean extract on days 7 and 14 could decrease the expression of TNF-α and increase TGF-β of the treatment group. Therefore, with an increased TNF-α apposition process, relapse after orthodontic treatment can be prevented to accelerate the orthodontic treatment.
Keywords: Osteoclast, Osteoblast, Estrogen, Epicatechin, Cytokine.
Orthodontic treatment is a way to move a malposition tooth to an appropriate position. Giving external mechanical forces in orthodontic tooth movement influences the remodeling of the periodontal ligament and alveolar bone1,2. These external mechanical forces induce neurotransmitters, cytokines, and growth factors, which send signals to pressure-related osteoclasts, causing bone resorption3-5. When an external mechanical force is applied to the pressure area of the periodontal membrane, osteoclasts are induced to resorb the alveolar bone. As a result, the tooth moves 6. Excessive bone resorption is an unwanted side effect of orthodontic treatment 7,8. The expression of the tumor necrosis factor (TNF) α will be induced by mechanical force in orthodontic tooth movement along with IL-1 to stimulate the activity of mature osteoclasts and withdraw other monocytes9-11. Additionally, lymphocyte and endothelial cell production of systemic RANKL are aided by TNF-α. Prostaglandin and TNF-α play a significant role in the maturation of osteoclasts12-14.
One of the most important factors in bone formation is the transforming growth factor beta TGF-β, which balances the dynamic processes of bone formation and resorption 15. By limiting the lifespan of osteoclasts through the promotion of apoptosis mediated by TGF-β, estrogen replacement may prevent excessive bone loss 7-16. Osteoblast and other cell production of TGF-β are effectively regulated by estrogen. By adjusting the levels of TGF-β in adults during OTM, natural remedies for the promotion of this mechanism could be a novel and useful therapeutic strategy for enhancing bone remodeling 4,6,17.
Phytoestrogen, a plant substrate with estrogen-like activity, is a form of estrogen replacement from plants 18,19. Epicatechin is one type of catechin, a polyphenolic compound in the flavonoid family 20-21. A few subsidiaries of cacao beans have been perceived to emphatically affect bone remodeling without promoting any side effects 22,23. It has previously been demonstrated that epicatechin improves osteoblast proliferation 4,23.
The aforementioned description raises a problem of whether cacao bean extract (Theobroma cacao L.) can affect the expression of TNF-α and TGF-β on orthodontic tooth movement at the pressure side of the alveolar bone. This study aims to determine the role of cacao bean extract (Theobroma cacao L.) on the expression of TNF-α and TGF-β on the movement of orthodontic teeth in the alveolar bone. The previously mentioned description raises an issue of whether cacao bean extract (Theobroma cacao L.) can influence the expression of TNF-α and TGF-β on orthodontic tooth movement at the pressure side of the alveolar bone. This study expects to decide the role of cacao bean extract (Theobroma cacao L.) on the expression of TNF-α and TGF-β of orthodontic tooth movement in the alveolar bone.
The sample size was 36 male Wistar rats aged 12-16 weeks and weights from 200-300 grams were partitioned into the experimental and control categories. Healthy rats without abnormalities were separated into six categories and marked with names and characteristics, as follows:
Group 1: a) Category with a 7-day control consisting of 6 rats (C7), which were forfeited following seven days, getting orthodontic movement or not, and not getting cacao bean extract administration. b) Category with a 14-day control consisting of 6 rats (C14), which were forfeited following seven days, getting orthodontic movement or not, and not getting cacao bean extract administration.
Group 2: a) Category with a 7-day control consisting of 6 rats (C-7), which were forfeited following seven days, getting orthodontic movement, and not getting cacao bean extract administration. b) Category with a 14-day control consisting of 6 rats (C-14), which were forfeited following seven days, getting orthodontic movement, and not getting cacao bean extract administration.
Group 3: a) Category with a 7-day control consisting of 6 rats (Q7), which were forfeited following seven days, getting orthodontic movement, and getting cacao bean extract administration. b) Category with a 14-day consisting of 6 rats (Q14), which were forfeited following fourteen days, getting orthodontic movement, and getting cacao bean extract administration.
A tension gauge was utilized to measure the distal heading of 10 grams of orthodontic power to the maxillary molar of male Wistar rats utilizing a prefabricated closed stainless steel coil spring (Ormco® Glendora, USA). The experiments were approved by the Research Ethics Committee (No.1728/UN25.8/KEPK/DL/2022).
The most common way of making cacao bean extract begins with unfermented mass (link) which is then extracted using the maceration method. A total of 1 kg of cocoa beans was extracted using 96% ethanol solvent with a ratio of 1:4 material and solvent which was carried out for 3 days and covered with aluminum foil with occasional stirring. The results of the macerate were concentrated using a rotary evaporator for 2 hours at a temperature of 40-50. The final result was 10.3 grams of cocoa bean extract. The dose of cocoa bean extract given to each rat was 50 mg diluted in 2 ml of aqua.
The dose of gel from cacao bean extract is 80mg/ml. It was given in the sulcus gingiva of the Wistar rat. Formalin was employed to fix the dissected maxillaries, and EDTA was employed to decalcify them. Paraffin-embedded and dehydrated samples were employed. TNF-α and TGF-β were stained on tissue areas under 180 µm from the distal root’s furcation of the upper molar. After dewaxing, the sections were treated with antigen retrieval and blocked for endogenous hydrogen peroxidase. Then, TNF-α and TGF-β of secondary antibodies were incubated.
The expression of TNF-α and TGF-β in the pressure side area was distinct from that of other groups. To show that the data were normally distributed, the Shapiro-Wilk normality test was conducted (p>0.05). Meanwhile, to demonstrate that the data were homogeneous, the Levene test was conducted. One-way ANOVA analysis was conducted to determine whether the expression was significantly affected; p<0.05 indicates a significant result. This study was also examined immunohistochemically. Meantime, a digital camera (Optilab Advance Plus) was utilized to take photos of the area.
This experimental study observed 36 male Wistar rats aged 12-16. Their classification is as follows. (C) with an absence of treatment and movement of the maxillary molar tooth. (C-) with maxillary molar tooth movement however not getting treatment. (Q) with maxillary molar tooth movement and treatment utilizing cacao bean extract. On days 7 and 14, the results of each category were examined.
The homogeneity of the tested data was initially assessed using the Levene Test. With a value of 0.08, the TGF-β and TNF-α expressions of groups met the premise of homogeneity. The data for each variable was then tested for normalcy. Figure 1 depicts the normal distribution using a QQ Plot for data analysis of TGF-β and TNF-α expressions from each variable, with the scatterplot forming both a straight and diagonal line, indicating that the data assumption of TGF-β and TNF-α expression by immunohistochemical staining is normal. Following that, other statistical tests, such as the one-way ANOVA analysis, can be performed.
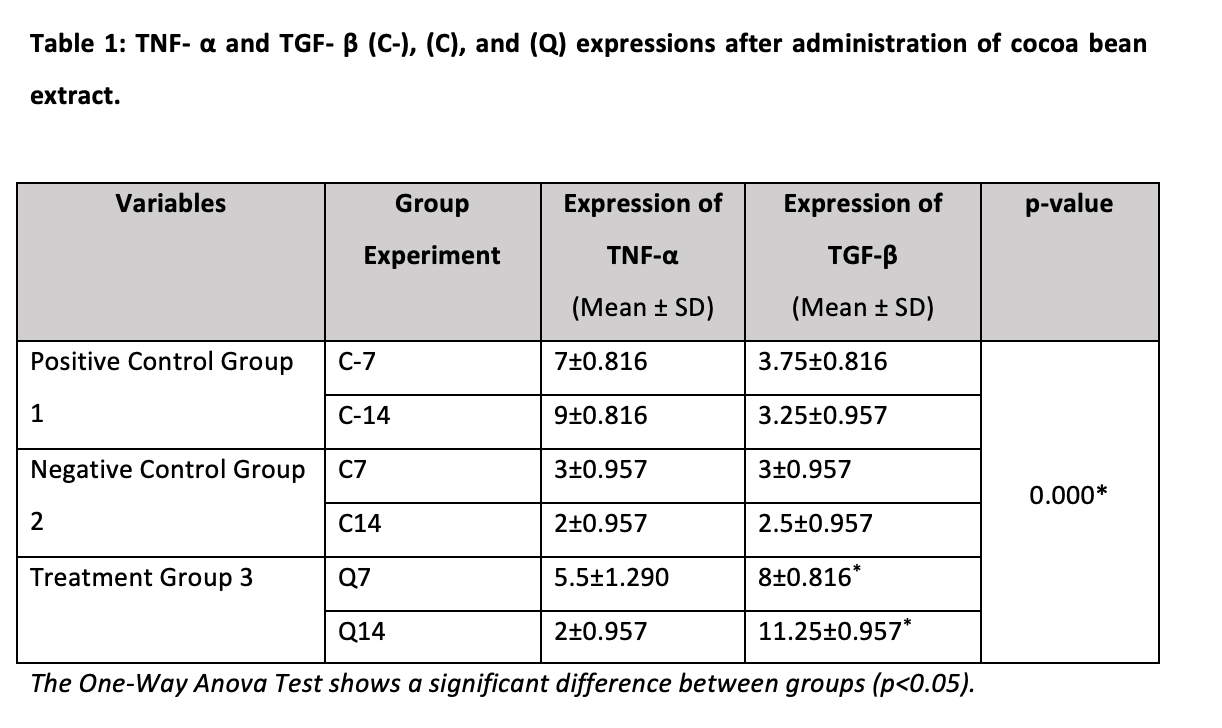
Table 1 shows that applying cocoa bean extract to the pressure side region of Q14 (2±0.957) may considerably reduce the mean expression of TNF-α in osteoclast cells, however, C-7 and C-14 do not. TGF-β, on the other hand, diminishes in osteoblast cells whereas C-7 and C-14 (3.75±0.816, 3.25±0.957) (p=0.000), as compared to other groups, but increases in treatment group Q14 (11.25±0.957*). The one-way ANOVA findings demonstrate a significant difference (p<0.05) between the negative control group (C), positive control group (C-), and treatment group (Q). On the pressure side, immunohistochemistry revealed a good reactivity to TGF-β and TNF-α (Figure 2).
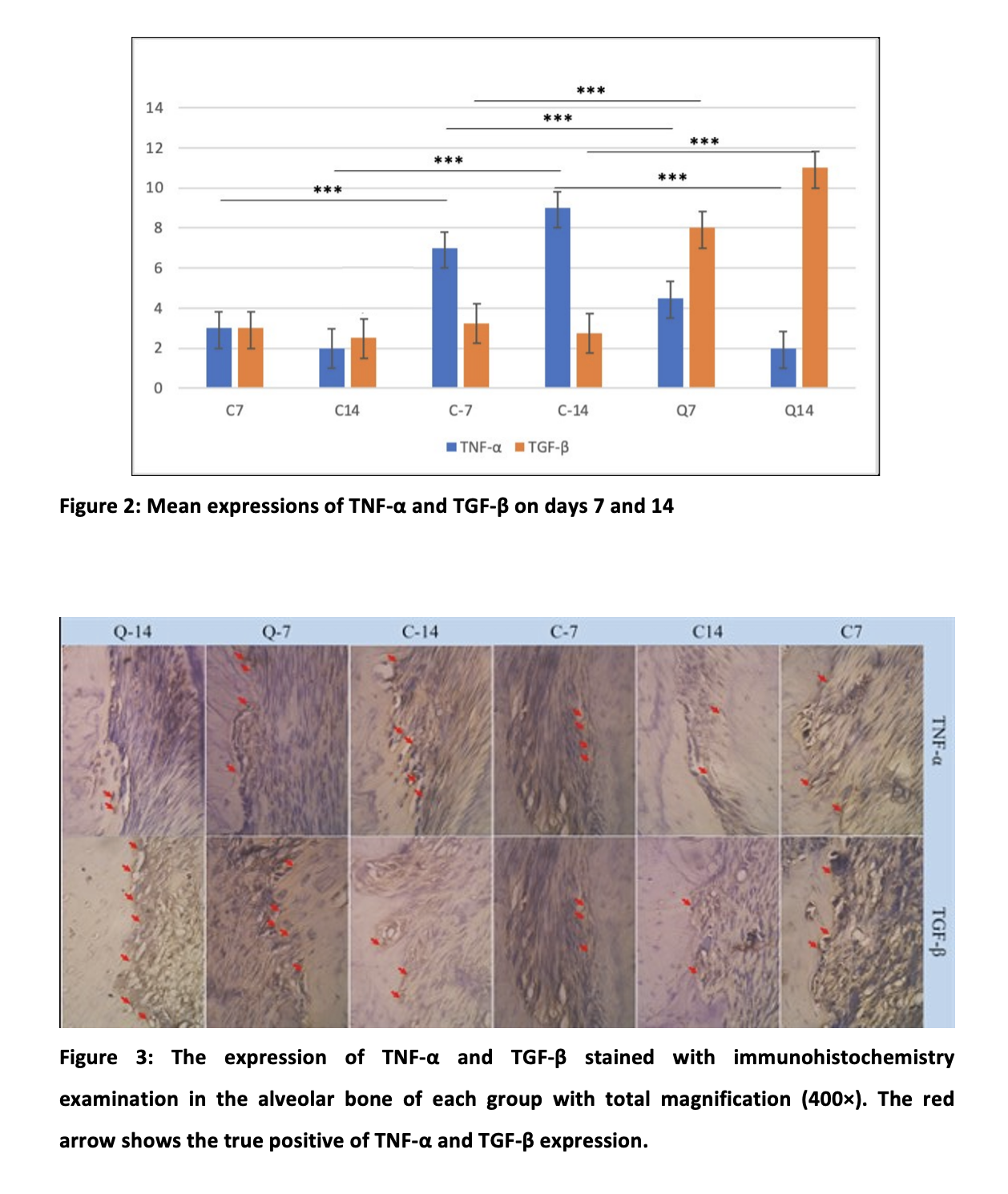
The immunohistochemistry findings in osteoclast cells that demonstrate positive results for TGF-β and TNF-α, expression by immunohistochemical staining is calculated for each group in this study based on different test factors. A one-way ANOVA technique compares different variables between groups for TGF-β and TNF-α. Wilk’s lambda is used to examine the data statistically based on the findings of the distribution normality test; a value of 0.000 (p<0.05) is achieved. This suggests that the impacts of the groups differ.
The researchers used orthodontic tooth movement in Wistar rats to explore how mechanical force loading induced the formation of osteoclast and odontoclast. TNF-α mediated osteoclast development during orthodontic tooth movement 24. The observation of osteoclast cells generated positive expression data of TNF-α and TGF-β by immunohistochemistry methods in the C7, C14, C-7, C-14, Q-7, and Q-14 represented in Figure 3.
TNF-α expression differs significantly between (C), (C-), and (Q) in Figure 1. On days 7 and 14, cocoa bean extract was seen to be used. TNF-α expression is reduced in this application. This finding implies that TNF- α plays a significant role in mediating pressure-side osteoclast development during orthodontic tooth movement. Root resorption is an unfavorable side effect of orthodontic therapy that occurs on occasion. Too much pressure force might induce root resorption 25. In the Wistar rat, root resorption can occur when 10 g of force is applied, and odontoclasts are present on the pressure side during tooth movement. TNF-α forms osteoclast by directly inducing macrophage osteoclast differentiation and stimulating RANKL expression in stromal cells 26.
In contrast, the result signifies a significantly different TGF-β expression among (C), (C-), and (Q). The application of cocoa bean extract was observed on days 7 and 14. This application shows that TGF-β expression increases. TGF-β regulates both osteoblasts and osteoclasts, which helps with bone remodeling. TGF-β is non-covalently attached to latency-associated protein (LAP) in the bone matrix, where it remains latent by concealing TGF-β’s receptor-binding domain. As a result, TGF-β stays dormant in the bone matrix and is released in response to osteoclast bone resorption. TGF-β activates bone mesenchymal lineage cells, causing them to develop into osteogenic osteoblasts and resorb surfaces 27.
Alveolar bone resorption by osteoclasts on the pressure side and new bone creation by osteoblasts on the tension side govern this remodeling. TNF-, which is expressed in the pressure side periodontal ligament, is critical in regulating distance tooth movement and osteoclastogenesis during OTM[4]. Epicatechin from cacao beans improves bone formation and inhibits bone resorption through cell signaling pathways that influence osteoblast differentiation. Epicatechin reduces osteoblast apoptosis and stimulates osteoblast cell proliferation and differentiation. The capacity of epicatechin to lower TNF- production is connected with its ability to inhibit osteoblast apoptosis 28.
The implication of giving cocoa bean extract can increase the apposition process and reduce the excessive resorption process in the alveolar bone so the remodeling process can run in balance because of its polyphenol content. It is hoped that with an increased apposition process, relapse after orthodontic treatment can be prevented so that orthodontic treatment can be accelerated.
The administration of cacao bean extract could decrease TNF-α and increase TGF-β. TNF-α. Moreover, this administration plays a significant role in osteoclast formation at the pressure side during orthodontic tooth movement. TGF-β, on the other hand, is expressed more by myofibroblasts, which could be a target for controlling mechanical signal transduction and tissue remodeling. The formation of bone and remodeled PDL fibers in this research has increased osteoblast and decreased osteoclast activities.
Thanks to all contributor for their participation in compiling this research.
All the authors at this moment declare that there is no conflict of interest.
This experimental study has received ethical approval from the Ethics Committee No.1765/UN25.8/KEPK/DL/2022.
DP did conceptualization, writing, review, and editing. RS did conceptualization, supervision, writing – review & editing. S also supervised and provided resources for the manuscript. AK has done data curation and formal analysis. DR supervision, investigation, and validation of data. DP provided the resources. RJ, SVF, FQAR, LN, SNF, and MM wrote the original draft and did data curation.
- Arina YMD, Ferdiansyah F, Rubianto M. The evaluation of mandibular bone density in chronic periodontitis models. Dent J (Majalah Kedokt Gigi) 2018;51:210. https://doi.org/10.20473/j.djmkg.v51.i4.p210-215.
- Li Y, Jacox LA, Little SH, Ko CC. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J Med Sci 2018;34. https://doi.org/10.1016/j.kjms.2018.01.007.
- Usui M, Onizuka S, Sato T, Kokabu S, Ariyoshi W, Nakashima K. Mechanism of alveolar bone destruction in periodontitis — Periodontal bacteria and inflammation. Jpn Dent Sci Rev 2021;57. https://doi.org/10.1016/j.jdsr.2021.09.005.
- Hernawati S, Sutjiati R, Sulistiyani, Martin M, Fadiyah SN. The Effect of Cacao Bean Extract on the Number of Osteoblasts on Orthodontic Tooth Movement. J Int Dent Med Res 2023;16(2):594–599.
- Sandra Sari D, Martin M, Maduratna E, Basuki Notobroto H, Mahyudin F, Sudiana K, et al. Combination adipose-derived mesenchymal stem cells-demineralized dentin matrix increase bone marker expression in periodontitis rats. Saudi Dent J 2023. https://doi.org/10.1016/j.sdentj.2023.07.019.
- Ogawa S, Kitaura H, Kishikawa A, Qi J, Shen WR, Ohori F, et al. TNF-α is responsible for the contribution of stromal cells to osteoclast and odontoclast formation during orthodontic tooth movement. PLoS One 2019;14. https://doi.org/10.1371/journal.pone.0223989.
- Jeon HH, Teixeira H, Tsai A. Mechanistic insight into orthodontic tooth movement based on animal studies: A critical review. J Clin Med 2021;10. https://doi.org/10.3390/jcm10081733.
- Li Y, Ling J, Jiang Q. Inflammasomes in Alveolar Bone Loss. Front Immunol 2021;12. https://doi.org/10.3389/fimmu.2021.691013.
- Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG, et al. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front Oral Biol 2015;18:1–8. https://doi.org/10.1159/000351894.
- Zhang F, Si M, Wang H, Mekhemar MK, Dörfer CE, Fawzy El-Sayed KM. IL-1/TNF-α inflammatory and anti-inflammatory synchronization affects gingival stem/progenitor cells’ regenerative attributes. Stem Cells Int 2017;2017. https://doi.org/10.1155/2017/1349481.
- Chu YF. Coffee: Emerging Health Effects and Disease Prevention. 2012. https://doi.org/10.1002/9781119949893.
- Hienz, Stefan A; Paliwal, Sweta, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res 2015;2015:1–10. https://doi.org/10.1016/8756-3282(85)90328-X.
- Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm Regen 2020;40. https://doi.org/10.1186/s41232-019-0111-3.
- Yasuda H. Correction to: Discovery of the RANKL/RANK/OPG system (Journal of Bone and Mineral Metabolism, (2021), 39, 1, (2-11), 10.1007/s00774-020-01175-1). J Bone Miner Metab 2021;39. https://doi.org/10.1007/s00774-021-01203-8.
- Sutjiati R, Narmada IB, Sudiana IK, Rahayu RP. The Inhibition of Relapse of Orthodontic Tooth Movement by NaF Administration in Expressions of TGF-β1, Runx2, Alkaline Phosphatase and Microscopic Appearance of Woven Bone. World Acad Sci Eng Technol Int J Med Heal Sci 2017;11:567–574.
- Xia J, Minamino S, Kuwabara K, Arai S. Stem cell secretome as a new booster for regenerative medicine. Biosci Trends 2019;13:299–307. https://doi.org/10.5582/bst.2019.01226.
- Alamsyah Y, Elianora D. Effect of Mangiferin (Mangifera Indica Linn) on the amount of osteoclast in the post-orthodontic treatment of bone Remodelling. Prep. Dent. To Approach Ind. Revolut. 4.0, UNMAS Press; 2019, p. 168–174.
- Min J, Yuan Z, Zhang Q, Lin S, Wang K, Luo J. Analysis of anti-osteoporosis function of chlorogenic acid by gene microarray profiling in ovariectomy rat model. Biosci Rep 2018;38. https://doi.org/10.1042/BSR20180775.
- Wang S, Ma Q, Xie Z, Shen Y, Zheng B, Jiang C, et al. An Antioxidant Sesquiterpene Inhibits Osteoclastogenesis Via Blocking IPMK/TRAF6 and Counteracts OVX-Induced Osteoporosis in Mice. J Bone Miner Res 2021;36. https://doi.org/10.1002/jbmr.4328.
- Ruso S, Pirman P. the Processing and Analysis of the Polyphenols Content of Cocoa Bean (Theobroma Cocoa L) and the Development As Functional Foods. Pros Semin Has Penelit 2018;2018.
- Pujiastuti P, Sakinah NN, Arina YMD, Wahyukundari MA, Praharani D, Sari DS. The potential of toothpaste containing Robusta coffee bean extract in reducing gingival inflammation and dental plaque formation. Dent J (Majalah Kedokt Gigi) 2023;56. https://doi.org/10.20473/j.djmkg.v56.i2.p109-114.
- Gabbay Alves TV, Silva da Costa R, Aliakbarian B, Casazza AA, Perego P, Pinheiro Arruda MS, et al. Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat Prod Res 2019;33. https://doi.org/10.1080/14786419.2017.1399381.
- Martin M, Sari DS, Mantika RA, Praharani D. Combination of Dental Pulp Stem-Cell Secretome and Robusta Coffee Bean Extract (Coffea canephora) in Enhancing Osteocalcin and Alkaline Phosphatase Expression in Periodontitis-Induced Wistar Rats. J Orofac Sci 2021;13. https://doi.org/10.4103/jofs.jofs_157_21.
- Loi F, Córdova LA, Pajarinen J, Lin T hua, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone 2016;86:119–130. https://doi.org/10.1016/j.bone.2016.02.020.
- Chaushu S, Klein Y, Mandelboim O, Barenholz Y, Fleissig O. Immune Changes Induced by Orthodontic Forces: A Critical Review. J Dent Res 2022;101. https://doi.org/10.1177/00220345211016285.
- Fu C, Shi R. Osteoclast biology in bone resorption: a review. STEMedicine 2020;1. https://doi.org/10.37175/stemedicine.v1i4.57.
- Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020;9. https://doi.org/10.3390/cells9092073.
- Babosova R, Mondockova V, Omelka R, Bauerova M, Galbavy D, Kalafova A, et al. The impact of flavonoid epicatechin on compact bone microstructure in rabbits. Biologia (Bratisl) 2020;75. https://doi.org/10.2478/s11756-019-00343-7.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
