By Kanwal Iqbal1, Mervyn Hosein 1, Saima Akram Butt1, Fizza Abidi 1
- Oral Pathology Department, Ziauddin University, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD12-4/013
How to cite: Iqbal K, Hosein M, Butt SA, Abidi F. Expression of Salivary HIF-1α Levels Among the Clinical Stages of Oral Submucous Fibrosis. Pak J Med Dent. 2023;12(4): 69-74. Doi: 10.36283/PJMD12-4/013
Background: Oral Submucous Fibrosis (OSMF) is defined as severe submucosal fibrosis and decreased vascularity, resulting in a compromised blood supply leading to hypoxia of tissues. The key biomarker involved is Hypoxia Inducible Factor-1α (HIF-1α). The objective of this research was to analyze the expression of HIF-1α in saliva samples of individuals across the clinical stages of OSMF and healthy controls.
Methods: This case-control study consisted of 120 participants, 60 patients had different clinical stages of OSMF and 60 healthy controls. Samples of saliva were collected and quantification of salivary samples was done using ELISA. Kolmogorov-Smirnov test assessed data normality. Kruskal Wallis test was applied to assess the association between salivary HIF-1α levels and clinical stages of OSMF. The Kendall Tau correlation test was used to assess the relationship between different stages of OSMF and salivary HIF-1α levels.
Results: The comparison of HIF-1α expression in different stages of OSMF and healthy controls was done. In comparison, it was found that HIF-1α expression increased significantly (p=0.00) in patients than controls. The expression was increased with stages, stage I (16.84 ± 9.74), stage II (19.51 ± 7.99), stage III (24.43 ± 7.49), and stage IV (28.92 ± 5.12), respectively. The odd ratio indicates for every progressive stage, the odds for high salivary HIF-1α levels increase by 1.63 times.
Conclusion: Levels of salivary HIF-1α showed increased expression with the progressive stages of OSMF. This variation in the levels of salivary HIF-1α suggests its possible role as an early non-invasive detective marker in the assessment of disease progression.
Keywords: Oral Submucous Fibrosis, Saliva, HIF-1α.
OSMF is a high-risk precancerous condition with a malignant transformation rate of about 7 to 30%, with a prevalence of about 0.03% to 6.42% 1. OSMF is differentiated by severe fibrosis of the lamina propria and deeper connective tissues of the oral mucosa2. More than 5 million people worldwide are suffering from OSMF according to the World Health Organization (WHO)3. Areca nut is a significant risk factor for OSMF as it has been identified in several studies4. Pakistan is one of the Southeast Asian countries with widespread use of areca nut and smokeless chewable tobacco. Due to these products being freely available in Pakistan, it increases OSMF incidence in this country5. According to a study done in rural Sindh, Pakistan, consumers of areca nuts and associated products were more likely to have OSMF. Additionally, studies on teenagers have documented an association between OSMF and areca nut usage, showing that 50- 79.6% of users developed OSMF6.
HIF-1α is a transcriptional activator of genes that modulates oxygen homeostasis and metabolic activation. It is a key regulator in sensing and responding to cellular oxygen levels7. HIF-1α contributes to angiogenesis in tumors by generating cell vesicles that facilitate cell-to-cell contact at a distance. Under hypoxia, HIF-1α interacts with hypoxia-responsive elements and becomes stable before translocating into the nucleus and it regulates the expression of several genes that enable cells to withstand and adapt to hypoxia8. In the initial stage of OSMF, there is increased expression of HIF-1α which induces the transcription of cytokines (such as TGF-β, PAI-1and PDGF), which increases the production of fibroblast and collagen and prevents collagen breakdown leading to fibrosis9. There is also an association between hypoxia and fibrosis in fibroblasts of kidney and lung as shown in literature 10, 11. Increased expression of HIF-1α and risk of progression to malignancy have been observed in OSMF. This may be due to OSMF connective tissue fibrosis and vasoconstriction, resulting in reduced blood flow to the local tissue environment. Therefore, it is believed to be a key element in the development of malignant transformation12.
HIF-1α is overexpressed in OSMF compared to controls and found statistically significant results, according to numerous studies proposed by several researchers. All these studies were based on OSMF tissue samples13, 14. Experiments utilizing a non-invasive method to assess salivary samples of patients with OSMF reported HIF-1α overexpression as compared to controls, suggesting that salivary HIF-1α levels can help identify the early stages of this disease15.
Based on the aforementioned findings, we proposed that HIF-1α can be a biomarker for early diagnosis of OSMF and it may help prevent the progression of this pre-cancerous disease in a population that is highly affected. The objective of this clinical study was to compare the different levels of HIF-1α in the saliva samples of patients with clinical stages of OSMF and controls.
The consecutive sampling technique was applied in this study. The sample size was estimated using OpenEpi software. There were 120 participants in the study which were divided into 2 groups: 60 clinically diagnosed with different stages of OSMF and 60 healthy controls. Both groups included men and women with a history of areca nut usage and smokeless chewable tobacco. The study did not include OSMF patients who had received steroid treatment or had persistent oral and systemic illnesses. This study was analyzed by Ziauddin University’s Research Ethics Committee (Reference code: 1840120KIOM).
All candidates were clinically assessed and all OSMF cases were identified using the clinical criteria put forward by More et al16 using the clinical staging. 5ml saliva samples were taken from all the candidates and placed in sterile falcon tubes using passive drool methods following the interview and clinical examination. A commercially available ELISA kit (SEA798Hu, USCN, USA) was used to determine salivary HIF-1α levels.
All data analysis was done using SPSS version 22. In terms of mean and standard deviation, demographic data and HIF-1α salivary concentrations were presented. The normal distribution of each variable was tested using the Kolmogorov-Smirnov test. Kruskal Wallis test was used to evaluate the association between salivary HIF-1α levels and clinical and functional stages of OSMF. The Kendall Tau correlation test was used to assess the relationship between different stages of OSMF and salivary HIF-1α levels. A p-value of below 0.05 was considered significant.
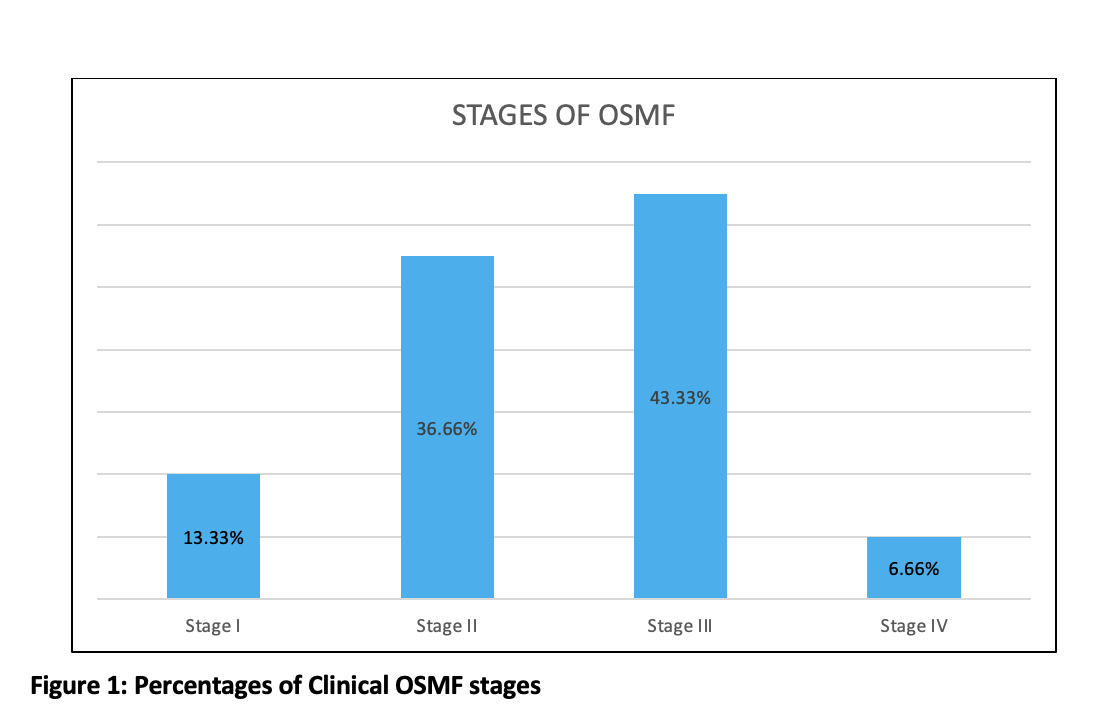
The study comprised 120 participants (60 individuals with different clinical stages of OSMF and 60 healthy controls). The proportion of male participants was 56.66% and female participants was 43.33% in the present study. The mean age for OSMF and healthy controls was 36.46 years and 31.41 years, respectively. Out of 60 OSMF patients, 8 (13.33%) were of stage I, 22 (33.33%) were of stage II, 26 (43.33%) patients of stage III, and 4 (6.66%) were of stage IV (figure 1).
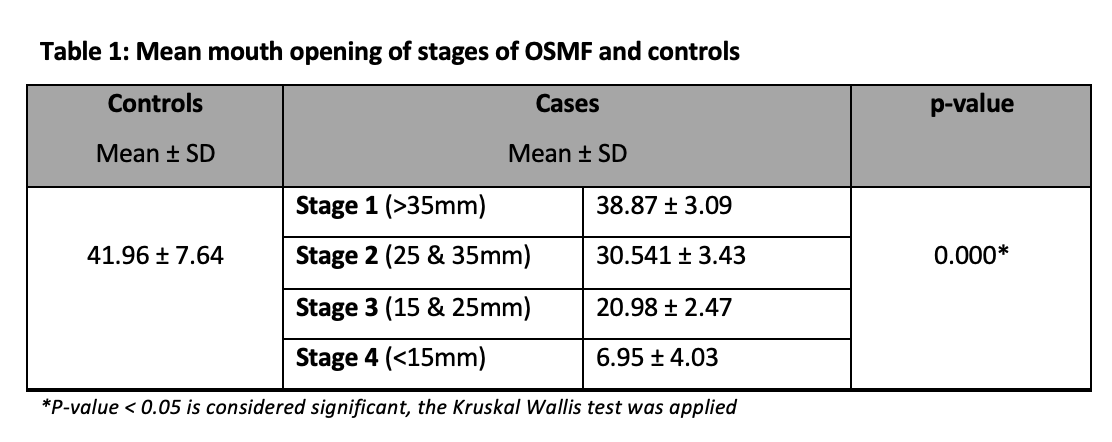
The mean mouth opening of different stages of OSMF and controls were recorded and showed a significant association, as shown in Table 1.
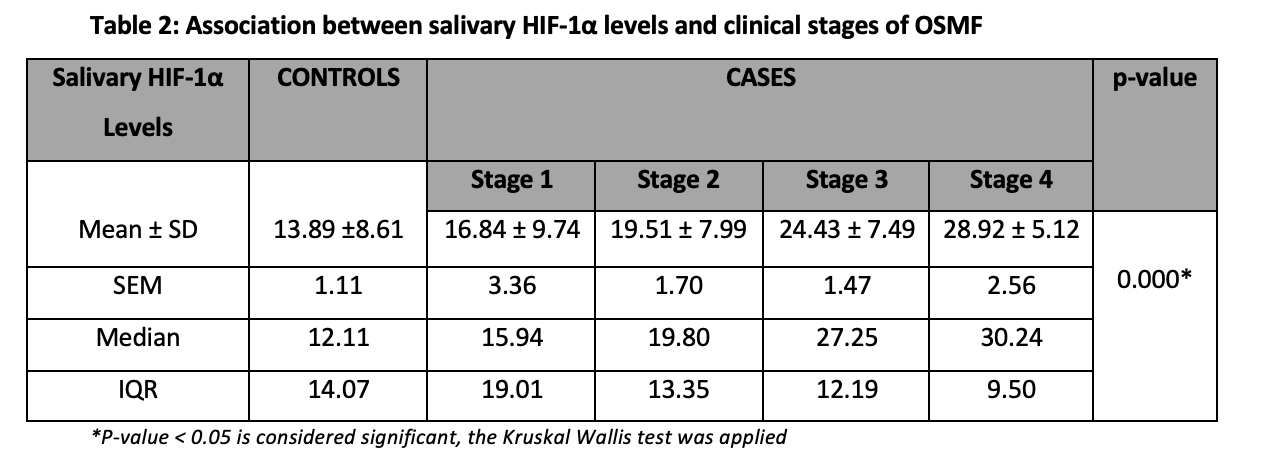
The mean salivary HIF-1α levels of different stages of OSMF patients and healthy controls were measured and clinical stages of OSMF showed significant association with salivary HIF-1α levels (p<0.05) (Table 2).
Kruskal Wallis test was applied to assess the association between salivary HIF-1α levels and clinical stages of OSMF and a significant association was observed (p=0.000) (Figure 2).

Figure 2: Box and Whisker plot depicting the increase in salivary HIF 1- α concentration at different stages of OSMF.
Also, significant positive correlations were observed between increasing salivary HIF-1α levels and advancing stages (Table 3).
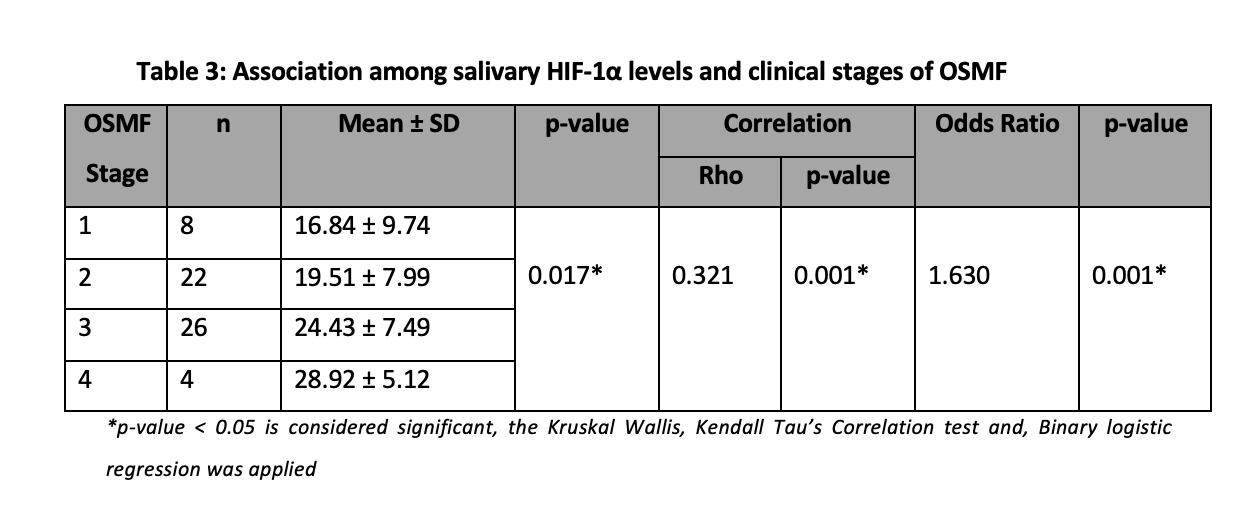
For the calculation of the Odd’s ratio, the salivary levels were categorized into high and low levels based on the sample median. The value of the odd’s ratio indicates 1.63 times increasing high odds of salivary levels. Therefore, for every progressive stage, the odds for high salivary HIF-1α levels increase by 1.63 times (Table 3).
In this study, the salivary expression of HIF-1α across clinical stages of OSMF was evaluated. The stages of OSMF i.e., stages I, II, III & IV clinically diagnosed according to the classification suggested by More et al. In this study, we were able to find a significant variation in levels of salivary HIF-1α in association with different stages of OSMF.
In our study, most patients were presented as stage III which suggests a lack of awareness among patients and delayed diagnosis. In another study conducted by Singh et al., the maximum number of patients in their study were of stage II (46.7%) followed by stage III, I, and IV17. In this study we found a significant association of disease severity with mouth opening i.e., there was a decrease in the inter-incisal mouth opening of the patients from stage I of OSMF to stage II, stage III, and stage IV respectively. The mean mouth opening of patients with different stages of OSMF was by studies reporting the mean mouth opening of patients with advanced stages of OSMF to be less than 15mm 18, 19.
Saliva samples were collected and utilized as the main analytical specimen in our study. Recently biomedical researchers have trying to evaluate the role of saliva as a key specimen in the detection and evaluation of multiple systemic and local pathological processes. Early detection of disease is key to decreasing morbidity, and mortality and preventing the complications of disease 21, 22. The role of saliva has been extensively reported not only because of its ease of availability and non-invasive nature of procedure but also due to the abundance of genetic material and proteins in saliva samples23, 24. Researchers are trying to establish the role of salivary samples as an early diagnostic tool that can be used at the chairside/bedside for the detection of oral as well as systemic diseases, 26. We assessed the levels of HIF-1α in the saliva samples of different clinical stages of OSMF patients and healthy controls.
The present study was designed with the presumption that due to fibrosis of connective tissue in OSMF, there is constriction of blood vessels followed by decreased flow of blood to the nearby tissues causing hypoxia which results in the activation of HIF-1α 27-29. In this study, we correlated the levels of salivary HIF-1α among the four clinical stages of OSMF and healthy controls using ELISA. We compared mean salivary HIF-1α levels in different clinical stages of OSMF with controls. The levels of salivary HIF-1α showed a rising trend from stage I to stage II to stage III to stage IV. We were able to find a significant variation in salivary HIF-1α levels between healthy individuals and different stages of OSMF (p<0.05) supporting our hypothesis that salivary expression of HIF-1α will progressively increase with OSMF stages. The results of this study indicated a rise in the levels of HIF-1α from healthy to diseased (OSMF patients). In the literature search, we found multiple studies that corroborate our findings and suggest the use of HIF-1α as a biomarker of progression. Hande et al. also evaluated HIF-1α expression in different grades of OSMF and concluded that expression increased significantly from normal oral mucosa (1.43±0.89) to no dysplasia (3.97±2.75) to low-grade dysplasia (4.93±1.76) to high-grade dysplasia (5.66±2.01)30.
Clinical and functional stages of OSMF showed significant association with salivary HIF-1α levels (p<0.05). In this study, significant positive correlations were also found between increasing HIF-1α levels and advancing stages. It was further observed that a direct significant relation between salivary HIF-1α and severity of disease exists suggesting the possible role of this protein in saliva as an early biomarker for detecting this disease and its progression.
This study has some limitations. This was a case-control study and the nature of the data did not permit us to recognize the pathophysiology of OSMF and its molecular relationship with salivary HIF-1α levels. We also did not perform microscopic analysis to assess the severity of fibrosis. To explore HIF-1α levels and associate them with soft tissue changes in OSMF, longitudinal studies can be helpful. Observational studies are important in evaluating salivary HIF-1α levels at different stages of the disease. The only method used to diagnose OSMF was a clinical examination in this study. Pathologists can stage OSMF more precisely and make a more accurate diagnosis by using invasive techniques such as “punch biopsy”. However, patient compliance is significantly impacted by such measures.
In this study, we found a significant association among the expression of HIF-1α levels in saliva among different stages of OSMF. Levels of salivary HIF-1α showed increased expression from healthy through the progressive stages of OSMF (I<II<III<IV). This variation in the levels of salivary HIF-1α suggests its possible role as an early non-invasive detective marker in the assessment of disease progression.
The authors would like to thank Mr. Moazzam Ali Shahid for his cooperation and valuable guidance during the laboratory procedure.
The authors declare no conflict of interest.
Ethical approval was taken from the Ethics Review Committee (ERC) of Ziauddin University, Karachi with the reference code (1840120KIOM).
KI did the conceptualization of the study, literature search, data collection, and writing the article. MH, SB, and FA did the proofreading and overall evaluation.
- Peng Q, Li H, Chen J, Wang Y, Tang Z. Oral submucous fibrosis in Asian countries. Journal of Oral Pathology & Medicine. 2020; 49(4):294-304. doi.org/10.1111/jop.12924
- More CB, Rao NR. Proposed clinical definition for oral submucous fibrosis. Journal of oral biology and craniofacial research. 2019; 9(4): 311-314. doi.org/10.1016/j.jobcr.2019.06.016
- Shih Y-H, Wang T-H, Shieh T-M, Tseng Y-H. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. International journal of molecular sciences. 2019;20(12):2940. doi.org/10.3390/ijms20122940
- Kondaiah P, Pant I, Khan I. Molecular pathways regulated by areca nut in the etiopathogenesis of oral submucous fibrosis. Periodontology 2000. 2019;80(1):213-24. doi.org/10.1111/prd.12266
- Raffat MA, Hadi NI, Hosein M, Zubairi AM, Ikram S, Akram Z. Differential expression of salivary S100A7 in oral submucous fibrosis. The Saudi Dental Journal. 2019;31(1):39-44. doi.org/10.1016/j.sdentj.2018.09.007
- Raffat MA, Hadi NI, Alghamdi O, Al-Aali KA, Al Deeb M, Abduljabbar T, et al. Expression of Salivary S100A7 Levels in Stage I Oral Submucous Fibrosis: A Clinical and Laboratory Study. Asian Pacific journal of cancer prevention: APJCP. 2020;21(4):1115-1119. doi: 10.31557/APJCP.2020.21.4.1115
- Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell metabolism. 2018;27(2):281-298. https://doi.org/10.1016/j.cmet.2017.10.005
- Albanese A, Daly LA, Mennerich D, Kietzmann T, Sée V. The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity. International Journal of Molecular Sciences.2021;22(1):268.doi.org/10.3390/ijms22010268
- Joseph I, Elizabeth J, Rao UK, Ranganathan K. Study of Hypoxia-inducible factor-2α expression in the malignant transformation of Oral submucous fibrosis. Journal of Oral and Maxillofacial Pathology: JOMFP. 2020;24 (1):33. doi: 10.4103/jomfp.JOMFP_42_19
- Zhao H, Han Y, Jiang N, Li C, Yang M, Xiao Y, Wei L, Xiong X, Yang J, Tang C, Xiao L. Effects of HIF-1α on renal fibrosis in cisplatin-induced chronic kidney disease. Clinical Science. 2021;135 (10):1273-1288. doi.org/10.1042/CS20210061
- Senavirathna LK, Huang C, Yang X, Munteanu MC, Sathiaseelan R, Xu D, et al. Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Scientific reports. 2018;8(1):1-16. DOI:10.1038/s41598-018-21073-x
- Chatterjee R, Ghosh B, Mandal M, Nawn D, Banerjee S, Pal M, et al. Pathophysiological relationship between hypoxia associated oxidative stress, Epithelial-mesenchymal transition, stemness acquisition, and alteration of Shh/Gli-1 axis during oral sub-mucous fibrosis and oral squamous cell carcinoma. European Journal of Cell Biology. 2021;100(1):151146. doi.org/10.1016/j.ejcb.2020.151146
- Pereira T, Surve R, Shetty S, Gotmare S. Qualitative expression of hypoxia-inducible factor-1α in malignant transformation of oral submucous fibrosis: An immunohistochemical study. Journal of Oral and Maxillofacial Pathology: JOMFP. 2020;24(1):106. doi: 10.4103/jomfp.JOMFP_234_19
- Shafiq N, Akram S, Khan TA, Nausheen N, Khan MA, Nasir U. Oral Submucous Fibrosis Patients Expressed Hypoxia Inducible Factor-1α (HIF-1α) in Saliva: A Biomarker for Detection of Malignant Transformation. PJMHS. 2022; 16(1).doi.org/10.53350/pjmhs22161798
- Iqbal et al K. Salivary Expression of HIF-1 α in Oral Submucous Fibrosis. PJMD. 2021;10(4):57-62. doi.org/10.36283/pjmd.v10i4.1546
- More CB, Rao NR, Hegde R, Brahmbhatt RM, Shrestha A, Kumar G. Oral submucous fibrosis in children and adolescents: Analysis of 36 cases. Journal of Indian Society of Pedodontics and Preventive Dentistry. 2020 ;38(2):190-199. DOI: 10.4103/JISPPD.JISPPD_173_20
- Singh P, Rai A, Dohare R, Arora S, Ali S, Parveen S, et al. Network‑based identification of signature genes KLF6 and SPOCK1 associated with oral submucous fibrosis. Molecular and clinical oncology. 2020;12(4):299-310. doi.org/10.3892/mco.2020.
- Panda S, Panda BK, Pattnaik B, Naik C, Dany SS, Avijeeta A. Prevalence of Oral Submucous Fibrosis in a Tertiary Care Hospital of Odisha-A Cross-Sectional Study.2020 ; 7(49), 2903-2906. DOI: 10.18410/jebmh/2020/594
- Rai A, Parveen S, Shree P, Ahmed T, Ali S, Kaur M, Sircar K, Sybil D, Chandra A. Salivary transforming growth factor beta in oral submucous fibrosis: A diagnostic and predictive marker. Journal of Cancer Research and Therapeutics. 2023. DOI: 10.4103/jcrt.jcrt_1929_22
- Chinnamurthy B, Jyothirmai K, Reddy RS, Ramesh T, Babu BA, Darna G. Saliva as a Diagnostic Tool in Forensic Odontology: Scope and Methods. Uttar Pradesh Journal of Zoology. 2023;30:95-104. DOI: 10.56557/UPJOZ/2023/v44i93497
- Chu HW, Chang KP, Yen WC, Liu HP, Chan XY, Liu CR, et al. Identification of salivary autoantibodies as biomarkers of oral cancer with immunoglobulin A enrichment combined with affinity mass spectrometry. Proteomics. 2023:2200321.doi.org/10.1002/pmic.202200321
- Errazquin R, Carrasco E, Del Marro S, Suñol A, Peral J, Ortiz J, Rubio JC, Segrelles C, Dueñas M, Garrido-Aranda A, Alvarez M. Early diagnosis of oral cancer and lesions in Fanconi anemia patients: a prospective and longitudinal study using saliva and plasma. Cancers. 2023;15 (6):1871. doi.org/10.3390/cancers15061871
- Song M, Bai H, Zhang P, Zhou X, Ying B. Promising applications of human-derived saliva biomarker testing in clinical diagnostics. International Journal of Oral Science. 2023;15(1):2. doi.org/10.1038/s41368-022-00209-w
- Liao C, Chen X, Fu Y. Salivary analysis: An emerging paradigm for non‐invasive healthcare diagnosis and monitoring. Interdisciplinary Medicine. 2023:e20230009. doi.org/10.1002/INMD.20230009
- Gug I, Tertis M, Ilea A, Chiș IA, Băbțan A-M, Uriciuc WA, et al. Salivary Biomarkers in Toxicology: An Update Narrative. Biomarkers in Toxicology: Springer; 2023: 647-673. https://doi.org/10.1007/978-3-030-87225-0_70-1
- Ngamchuea K, Chaisiwamongkhol K, Batchelor-McAuley C, Compton RG. Chemical analysis in saliva and the search for salivary biomarkers–a tutorial review. Analyst. 2018;143(1):81-99. DOI:10.1039/C7AN01571B
- Dalmia A, Hazarey V, Talkal R, Ganvir S, Purohit HJ, Gupta S, Pimpale A. Role of Hypoxia Inducible Factor-1α messenger RNA expression in malignant transformation of oral submucous fibrosis: A RT-PCR study. Translational Research in Oral Oncology. 2016; 1:1-5 doi.org/10.1177/2057178X16655554
- Chaudhary M, Bajaj S, Bohra S, Swastika N, Hande A. The domino effect: Role of hypoxia in malignant transformation of oral submucous fibrosis. Journal of oral and maxillofacial pathology: JOMFP. 2015; 19(2):122. doi: 10.4103/0973-029X.164519
- Tilakaratne WM, Iqbal Z, Teh MT, Ariyawardana A, Pitiyage G, Cruchley A, Stewart JE, Hagi‐Pavli E, Lalli A, Waseem A, Parkinson EK. Upregulation of HIF‐1α in malignant transformation of oral submucous fibrosis. Journal of Oral Pathology & Medicine. 2008; 37(6):372-377. doi.org/10.1111/j.1600-0714.2007.00625.x
- Hande AH, Chaudhary MS, Gadbail AR, Zade PR, Gawande MN, Patil SK. Role of hypoxia in malignant transformation of oral submucous fibrosis. Journal of Datta Meghe Institute of Medical Sciences University. 2018;13(1):38. DOI: 10.4103/jdmimsu.jdmimsu_40_18.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
