By Saba Zaidi1, Naveed Uddin Ahmed1
- Assistant Professor, Department of Neurology at Liaquat national hospital, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD12-3/009
How to cite: Zaidi S, Ahmed NU. Relationship Between Decremental Response on Repetitive Nerve Stimulation and Amyotrophic Lateral Sclerosis Functional Rating Scale. Pak J Med Dent. 2023;12(3): 49-53. doi: 10.36283/PJMD12-3/009
Background: Amyotrophic Lateral Sclerosis (ALS), is a progressive degenerative disease of the motor neuronal cells, and its clinical presentation includes upper motor neuron symptoms, lower motor neuron symptoms, bulbar symptoms, or a combination of them all. This study aimed to measure the association between decrements in Repetitive Nerve Stimulation (RNS) and the overall functional status of ALS patients.
Methods: In this cross-sectional study, routine RNS was performed, and its association was measured with the demographic data, disease onset, and ALS functional rating scales (ALSFRS) in 20 patients having ALS. RNS was performed and patients were divided into RNS-positive and RNS-negative groups. ALSFRS was used to assess disease severity, statistical analysis was done using SPSS, and the student t-test was used for comparison among groups.
Results: Twenty ALS patients (mean age: 55.85±11.18 years; 65% males) were enrolled. The patients belonged to the following professions: laborers (20%), shopkeepers (20%), homemakers (10%), retired from work (10%), and teachers (5%). The mean duration of illness was 14.00±12.98 months. No significant difference was observed in RNS decremental responses or ALSFRS scores between patients with limb and bulbar onset. There was also no statistically significant association between RNS decrements and ALSFRS scores (p=0.975).
Conclusion: The association between decremental response to RNS with the clinical correlation of patients with ALS was statistically not significant. Future studies with larger and more diverse samples are needed to validate these findings and provide a more comprehensive understanding of the relationship between RNS decrement and ALS.
Keywords: Amyotrophic Lateral Sclerosis, Atrophy, Nerve Stimulation.
Amyotrophic lateral sclerosis (ALS), also termed Lou Gehrig disease, is a progressive degenerative disease of the motor neuronal cells of the cerebral cortex, brainstem, and spinal cord. The prognosis of ALS is overall weak with almost all patients succumbing to respiratory failure eventually1. There are variable clinical presentations of ALS including upper motor neuron symptoms (UMN), lower motor neuron symptoms (LMN), bulbar symptoms, or a combination of them all. Repetitive nerve stimulation (RNS) is not a routine study but often comes abnormal in patients with ALS1-3. The decrements in RNS studies are often present in patients with ALS and may have an underlying role in the disease progression. It was found in research that decremental responses in the RNS were commonly observed in ALS patients4.
Repetitive nerve stimulation (RNS) is a commonly used electrodiagnostic technique to diagnose neuromuscular junction disorders5. In normal individuals, CMAP amplitude remains stable or increases slightly with successive stimuli. In contrast, in patients with neuromuscular junction disorders, there is a progressive decrement in the amplitude of CMAP, reflecting the failure of neuromuscular transmission with repetitive stimulation6. There is growing evidence that RNS may be a useful tool in the diagnosis and monitoring of ALS. Studies have shown that up to 80% of ALS patients exhibit abnormal decremental responses to RNS, with the orbicularis oculi and trapezius muscles being the most commonly affected7,8. The RNS decrement has been suggested to be related to the involvement of motor neurons and the subsequent loss of neuromuscular junction integrity in patients with ALS9. Additionally, it has been shown in previous research that the onset of ALS didn’t affect decremental responses in RNS10,11.
Furthermore, several studies have investigated the relationship between RNS decrements and disease severity in ALS. In a study of 31 ALS patients, a significant correlation was found between the RNS decrement in the trapezius muscle and the ALS Functional Rating Scale-Revised (ALSFRS-R) score, a measure of overall disease severity12. Another study found that RNS decrements in the orbicularis oculi muscle were associated with a more rapid disease progression, as measured by the time to reach significant respiratory dysfunction13. Despite these findings, the role of RNS in the diagnosis and monitoring of ALS remains controversial. To further characterize the role of RNS in patients with ALS, we conducted a cross-sectional study that aimed to measure any associations between decrements in RNS and the overall functional status of the enrolled ALS patients.
A total of 20 participants diagnosed with ALS were enrolled in this cross-sectional study after obtaining ethical approval from Liaquat National Hospital. Informed consent was obtained from all participants before data collection. Participants were identified during regular clinical visits and were included in the study if they fulfilled the following inclusion criteria: (1) any laboratory or clinically (according to El- Escorial diagnostic criteria) proven case of ALS, (2) age of 18+ years. Participants with comorbid neuromuscular junction disorders, myopathies, neuropathies, or variants of ALS like familial ALS, primary lateral sclerosis, progressive bulbar palsy, progressive muscular atrophy, or Hirayama were excluded from this study. Individuals under the age of 15 and those having familial history were also excluded to avoid misclassification with familial motor neuron disease resembling ALS.
During repetitive nerve stimulation (RNS), surface electrodes were attached to measure compound muscle action potential (CMAP) while keeping a constant skin temperature of 32 Celsius. Furthermore, RNS was undertaken in the following muscles and their associated nerves: deltoid (axillary nerve), trapezius (accessory nerve), and orbicularis oculi (facial nerve). The RNS was recorded by providing 10 counts of stimuli (each with a frequency of 3 Hertz) to the nerve of interest. The decrement in RNS was defined as a reduction in the amplitude of the CMAP for the 5th response to the 1st stimulus. A decrease of ≥10 % was considered statistically significant. When the decrease was > 10%, RNS was repeated after 10 seconds of isometric exercise with evaluation for repair (improvement of the absolute size of decrease by ≥2%).
A convenient sampling technique was employed for outpatient visits, and a thorough clinical interview was done for the diagnosis and duration of the illness. All patients underwent nerve conduction studies (NCS), electromyography (EMG), and repetitive nerve stimulation (RNS) tests. The participants were divided into two major groups: RNS positive (with decremental response to RNS) and RNS negative (no decremental response to RNS). To gauge the severity of the disease process and overall quality of life, ALS functional rating scale (ALSFRS) was used. The statistical analysis was done on the SPSS version 20 and student t-test was used for comparison among groups. An alpha value of 0.05 was selected arbitrarily and in accordance, a p-value < 0.05 was determined statistically significant.
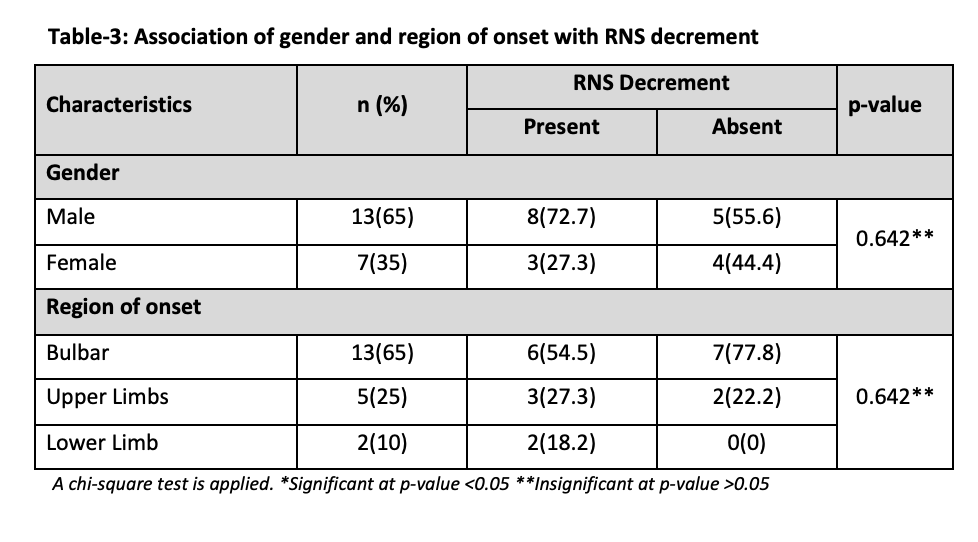
After initial screening, a total of 20 patients were enrolled in our study who had met the diagnostic criteria for ALS. The mean age was 55.85±11.18 years. With respect to gender, thirteen (65%) were males and seven (35%) were females. The patients belonged to the following professions: laborer (n=4; 20%), shopkeepers (n=4; 20%), homemakers (n=2; 10%), retired from work (n=2; 10%), and teacher (n=1; 5%). Among participants, four (20%) of them gave a history of electric shock exposure. The mean duration of illness was 14.00±12.98 months. There was no significant difference in the means of decremental responses in RNS and ALSFRs between patients with a limb (n=7) and bulbar onset (n=13) motor neuron diseases. Moreover, our study showed no statistically significant association between decrements in RNS and scores of ALSFRS (p=0.975) (Table 1).
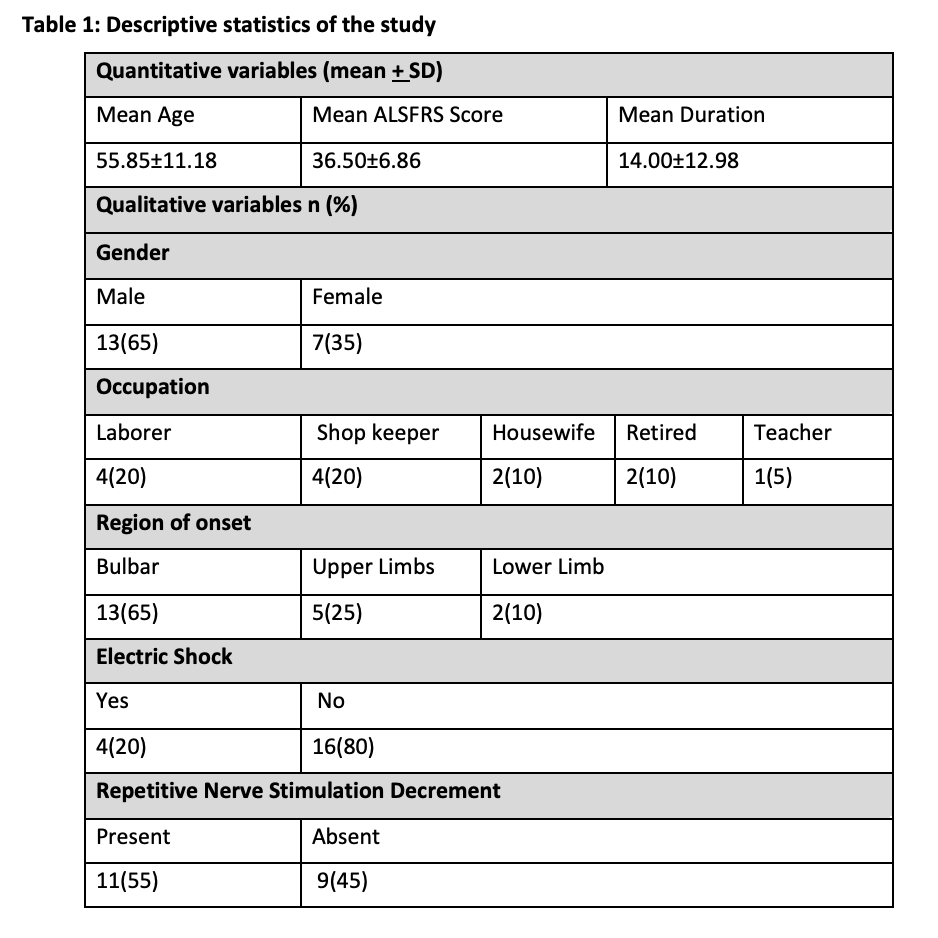
Among the participants, 55% showed RNS decrement, and 45% showed no decrement. There was no significant difference in the means of decremental responses in RNS and ALSFRs between patients with a limb (n=7) and bulbar onset (n=13) motor neuron diseases. Moreover, our study showed no statistically significant association between decrements in RNS and scores of ALSFRs (p=0.975).
The mean differences in different characteristics with respect to RNS decrement, including age in years, ALSFRS score, and duration of illness are presented in Table 2. Student t-test was applied, and all p-values were found to be insignificant (p>0.05).
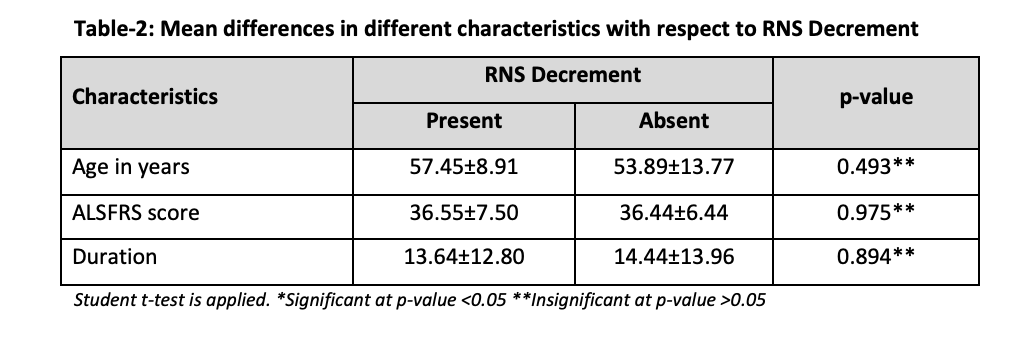
The association of different characteristics with RNS decrement, including gender, and region of onset are presented in Table 3. The chi-square test was applied, and all p-values were found to be insignificant (p>0.05). This suggests that there is no significant association between these factors and RNS decrement in ALS patients.
ALS is a progressive neurodegenerative disorder that primarily affects the upper and lower motor neurons, leading to muscle weakness, atrophy, and paralysis. It has shown that neuromuscular junction (NMJ) also gets affected other than UMNs and LMNs in patients with ALS. The primary cause behind NMJ pathophysiology might be collateral nerve terminal sprouting7. The decremental picture in low-frequency RNS among patients with ALS (predicted to show NMJ involvement) was observed across major nerves across the body, like the accessory, axillary, radial, median, ulnar, and facial nerves. The accessory nerve of the trapezius muscle showed the highest positive rate of decremental response to RNS whereas the facial nerve had the smallest positive rate in this regard8,9,11-13.
In a study, it was observed that RNS decrements were significantly greater in the median nerves of patients with ALS in comparison to the control group. In a further subgroup analysis, the authors added that RNS decrement was significantly greater in the cervical region‐onset group as compared to any other region‐onset groups14,15. Cui et al. showed that the decremental pattern in low RNS was significantly greater in limb-onset patients compared to bulbar onset patients with ALS16. Our study showed that those with a decremental response to RNS have the disease onset in limbs more than the bulbar region which is similar to the available literature. Moreover, the magnitude of decremental response in RNS was greater among the patients with ALS having rapidly progressive disease and muscle atrophy when compared with the ones having overall slowly progressive symptoms or lesser muscle atrophy. Hu et al analyzed that in degenerating axons in ALS patients, the more serious the axon damage, the more significant the decremental response in RNS. However, disease progression was not affected by the decremental response17.
However, in our study, no significant associations were found between low-frequency RNS results and the total duration of disease or ALS functional score among patients, the reason behind this is that the patients enrolled in the study were at different stages of their disease18. Another study showed a greater decrement in patients with ALS having major characteristics of atrophy and fasciculations when compared with other disease manifestations19. It has been stated that CMAP decremental responses in RNS were commonly found in ALS patients, suggestive of NMJ abnormalities20. Overarchingly, decrements in CMAP across patients with ALS had been predicted to arise from axonal conduction failure, neuronal degeneration, or at the NMJs of regenerating neurons due to collateral sprouting. RNS studies are an important tool in the diagnosis and differentiation of patients with ALS into specific subgroups that might benefit from differential management.
This study had certain limitations, the sample size being relatively small, which may limit the generalizability of the findings. Additionally, the study population consisted of patients from a specific region mostly from Karachi-one of the rural cities of Pakistan. This introduces the possibility of geographical bias, as different populations may have varied genetic backgrounds, environmental factors, or healthcare access that could influence the study results. Acknowledging this limitation is important to ensure that readers understand the context and generalizability of the findings. Another important factor is the technique to perform RNS which may produce potential measurement errors or interrater reliability issues. This area needs further improvement. Future studies with larger and more diverse samples are needed to validate these findings and provide a more comprehensive understanding of the relationship between RNS decrement and ALS.
RNS could be an important tool in characterizing the clinical and functional parameters of ALS, even though not routinely used in the analysis of patients with the disease. Nevertheless, the study did not show any statistically significant association between a decrease in RNS measurements and the overall clinical symptoms and functionality of patients with ALS.
We want to acknowledge the Neurophysiology Department of Liaquat national hospital for their help in performing the additional task for research purposes.
All authors confirm that there is no conflict of interest.
The institutional approval of the research study was attained by the Ethical Review Committee at Liaquat National Hospital.
Written consent was taken from all participants.
SZ was the principal investigator who designed, wrote, and proofread the manuscript. NA proofread the manuscript.
- Williams UE, Philip-Ephraim EE, Oparah SK. Multidisciplinary Interventions in Motor Neuron Disease. J Neurodegener Dis. 2014; 2014:435164. doi: 10.1155/2014/435164.
- Brown GL, Harvey AM. Neuro-muscular transmission in the extrinsic muscles of the eye. J Physiol. 1941 Mar 25;99(3):379-399. doi: 10.1113/jphysiol. 1941.sp003910.
- Mulder Dw, Lambert Eh, Eaton Lm. Myasthenic syndrome in patients with amyotrophic lateral sclerosis. Neurology. 1959 Oct;9: 627-631. doi: 10.1212/wnl.9.10.627.
- Sun XS, Liu WX, Chen ZH, Ling L, Yang F, Wang HF, Cui F, Huang XS. Repetitive Nerve Stimulation in Amyotrophic Lateral Sclerosis. Chin Med J (Engl). 2018 Sep 20;131(18):2146-2151. doi: 10.4103/0366-6999.240798.
- Denys EH, Norris FH Jr. Amyotrophic lateral sclerosis. Impairment of neuromuscular transmission. Arch Neurol. 1979 Apr;36(4):202-105. doi: 10.1001/archneur.1979.00500400056008.
- Bernstein LP, Antel JP. Motor neuron disease: decremental responses to repetitive nerve stimulation. Neurology. 1981 Feb;31(2):204-207. doi: 10.1212/wnl.31.2.202.
- Killian JM, Wilfong AA, Burnett L, Appel SH, Boland D. Decremental motor responses to repetitive nerve stimulation in ALS. Muscle Nerve. 1994 Jul;17(7):747-754. doi: 10.1002/mus.880170708.
- Zhang D, Zhao Y, Yan C, Cao L, Li W. CMAP decrement by low-frequency repetitive nerve stimulation in different hand muscles of ALS patients. Neurol Sci. 2019 Dec;40(12):2609-2615. doi: 10.1007/s10072-019-04027-7.
- Fu LL, Yin HX, Liu MS, Cui LY. Study on variation trend of repetitive nerve stimulation waveform in amyotrophic lateral sclerosis. Chin Med J (Engl). 2019 Mar 5;132(5):542-550. doi: 10.1097/CM9.0000000000000117.
- Wang Y, Xiao Z, Chu H, Liang J, Wu X, Dong H, Yan Y, Lu Z. Correlations between slow-rate repetitive nerve stimulation and characteristics associated with amyotrophic lateral sclerosis in Chinese patients. Journal of Physical Therapy Science. 2017;29(4):737-743.
- Alanazy MH, Hegedus J, White C, Korngut L. Decremental responses in patients with motor neuron disease. Brain and Behavior. 2017 Nov;7(11):e00846.
- Iwanami T, Sonoo M, Hatanaka Y, Hokkoku K, Oishi C, Shimizu T. Decremental responses to repetitive nerve stimulation (RNS) in motor neuron disease. Clin Neurophysiol. 2011 Dec;122(12):2530-2536. doi: 10.1016/j.clinph.2011.05.019.
- Yamashita S, Sakaguchi H, Mori A, Kimura E, Maeda Y, Hirano T, Uchino M. Significant CMAP decrement by repetitive nerve stimulation is more frequent in median than ulnar nerves of patients with amyotrophic lateral sclerosis. Muscle Nerve. 2012 Mar;45(3):426-428. doi: 10.1002/mus.22301.
- Shefner JM, Al-Chalabi A, Baker MR, Cui LY, de Carvalho M, Eisen A, Grosskreutz J, Hardiman O, Henderson R, Matamala JM, Mitsumoto H, Paulus W, Simon N, Swash M, Talbot K, Turner MR, Ugawa Y, van den Berg LH, Verdugo R, Vucic S, Kaji R, Burke D, Kiernan MC. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020 Aug;131(8):1975-1978. doi: 10.1016/j.clinph.2020.04.005.
- Sanders DB. Clinical neurophysiology of disorders of the neuromuscular junction. J Clin Neurophysiol. 1993 Apr;10(2):167-180. doi: 10.1097/00004691-199304000-00004.
- Cui LY, Liu MS, Tang XF. Single fiber electromyography in 78 patients with amyotrophic lateral sclerosis. Chin Med J (Engl). 2004 Dec;117(12):1830-3.
- Hu F, Jin J, Kang L, Jia R, Qin X, Liu X, Liu X, Liu C, Wang L, Zhang R, Dang J. Decremental responses to repetitive nerve stimulation in amyotrophic lateral sclerosis. European Neurology. 2019 Feb 1;80(3-4):151-156.
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001 May 31;344(22):1688-1700. doi: 10.1056/NEJM200105313442207.
- Amin Lari A, Ghavanini AA, Bokaee HR. A review of electrophysiological studies of lower motor neuron involvement in amyotrophic lateral sclerosis. Neurol Sci. 2019 Jun;40(6):1125-1136. doi: 10.1007/s10072-019-03832-4.
- Shang L, Chu H, Lu Z. Can the Large-Scale Decrement in Repetitive Nerve Stimulation Be Used as an Exclusion Criterion for Amyotrophic Lateral Sclerosis? Front Neurol. 2020 Feb 28; 11:101. doi: 10.3389/fneur.2020.00101.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
