By Kaneez Zehra 1, Samina Shamim2, Maryam Gohar3, Ayesha Siddiqi4, Ashok Kumar3
- Consultant Nephrologist, Department of Nephrology, Dr. Zia Uddin University Hospital, Karachi, Pakistan
- Senior Registrar, Department of Nephrology, Dr. Zia Uddin University Hospital, Karachi, Pakistan
- Consultant Pulmonology, Department of Pulmonology, Dr. Zia Uddin University Hospital, Karachi, Pakistan
- Research Associate, Department of critical care medicine, Dr. Zia Uddin University Hospital, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD12-3/005
How to cite: Zehra K, Shamim S, Gohar M, Siddiqi A, Kumar A. Complications of Acute Kidney Injury in Critically Ill COVID-19 Patients. Pak J Med Dent. 2023;12(3): 23-28. doi: 10.36283/PJMD12-3/005
Background: Various manifestations of COVID-19, and the occurrence of Acute Kidney Injury (AKI) in critically ill patients have been associated with higher mortality rates. This study aimed to find the outcome and complications of Acute Kidney Injury in critically ill COVID-19 Patients.
Method: This single-center study is based on the Kidney Disease Improving Global Outcomes (KDIGO) renal staging to ascertain the frequency and outcomes of patients(n=200) in the intensive care unit (ICU) with AKI and COVID-19 in the initial pandemic in Karachi. Study conducted between April 2020 – July 2021, in the ICU with critical COVID-19 patients at Ziauddin Hospital Clifton, Karachi. The variables employed to assess severity and outcomes were ventilation organ support, use of vasoactive therapy, need for renal replacement therapy (RRT), and patient status at ICU and/or hospital discharge.
Results: Two hundred patients were enrolled in the study, most were males (70%) with a mean age of 65 + 13 years. The frequency of AKI as per KDIGO stages was 43.5%, 13.5%, and 43% for AKI stages 1, 2, and 3, respectively. Forty-six patients (23%) required RRT. Among 200 patients, 75 % were known hypertensive and 45% were diabetics. Our results demonstrated that the overall mortality was 58.5% of patients, while the mortality rate of those who received RRT was 78.3%.
Conclusion: COVID-19 with AKI in critically ill patients was associated with worsened disease processes and a higher mortality rate. Most patients were elderly, hypertensive, and diabetics. The mortality rates were higher with higher stages of AKI and those needing RRT.
Keywords: COVID-19, Acute Kidney Injury, Renal Replacement Therapy.
With the global outbreak of coronavirus disease 19 (COVID-19), the world has been overwhelmed with the evolving and ever-increasing severity that has accompanied this disease with 450,229,635 confirmed cases and 6,019,085 deaths worldwide at the time of writing this manuscript as recorded by the World Health Organization1. Among the plethora of manifestations of COVID-19 that have been identified, the occurrence of acute kidney injury (AKI) in critically ill patients has been associated with higher mortality rates. Acute respiratory distress syndrome (ARDS) due to any cause is associated with a higher rate of complication including AKI with incidence reported in previous studies around 35%. ARDS secondary to COVID-19 is associated with much worse clinical outcomes and complications with reported incidence of AKI being as high as 59%2,3.
The renal injury presented in COVID-19 is considered multifactorial; is directly affected by the virus, and indirectly by the angiotensin-converting enzyme II (ACE II) pathway as well as a sequela of severe disease courses including sepsis, MOD4,5. A histopathological study exploring the renal tissues of six elderly COVID-19 patients’ postmortem, in China, showed that SARS-CoV-2 directly facilitates AKI development by inflicting tubular damage4. A meta-analysis in China showed the incidence of AKI with COVID-19 patients to be 11.6% and escalated up to 50% in patients admitted to the intensive care unit (ICU), observing an overall fatality rate of 20.3% associated with severe disease 6. The COVID-19 nephrology compendium stated highly variable statistics for the incidence of AKI across three continents ranging from 0.5 – 46% 6,7.
There are various risk factors associated with AKI in COVID- 19 including hypovolemia, ARDS, sepsis, hemodynamic instability, cytokine storm syndrome, drugs, and covid-19 virus that led to collapsing GN and direct tubular injury. The presence of acute proximal tubular injury, peritubular erythrocyte aggregation, and glomerular fibrin thrombi with the ischemic collapse in the autopsy of patients with covid-19 have been reported. It’s also reported endothelial damage, hemosiderin deposition, pigment casts related to rhabdomyolysis, and inflammation. Covid-19 infection also led to such histopathological changes as mentioned above without any evidence of renal failure raising the possibility of subclinical kidney injury8.
There has been ample data gathered on AKI in COVID-19 patients worldwide, and the heterogeneity in it is evident. In Pakistan, limited data exist on AKI and COVID-19 with single-center studies 9–11. Considering the inconsistency mentioned above, our study aims to be a drop in establishing a pooled data set for further research. Here, we highlight the incidence of AKI as a severe complication of COVID-19 and the significant impact on morbidity and mortality of the patient. Therefore, the objective of this study was to find the frequency and outcome of Acute Kidney Injury (AKI) in critically ill COVID-19 patients.
After approval from the Ethical Review Committee (ERC)(Reference number 2670920KZNEP), the study was carried out in Dr. Ziauddin Hospital, Clifton Campus which is a tertiary care modern healthcare hospital. Available medical records of all patients hospitalized with COVID-19 between April 2020 till July 2021 were reviewed retrospectively.
This was a single-center, retrospective cross-sectional study carried out in the ICU at Ziauddin Hospital Clifton, in which all patients(n=200) admitted with COVID-19 (diagnosed on the basis of COVID PCR or radiological evidence) with Acute Kidney Injury (AKI) were included while patients with known chronic kidney disease and pregnant females were excluded from the study. Demographic and clinical data including age, gender, comorbidities, the need for mechanical ventilation or vasoactive support, urine output, the need for renal replacement therapy, patient outcome, and renal function at discharge were recorded. Laboratory data including confirmatory tests of COVID-19 and biochemical parameters including urea and creatinine were taken to establish the diagnosis of COVID-19 with AKI.
The diagnosis of AKI was made on the basis of serum Creatinine or urine output, as per the consensus definition and criteria for AKI by the Kidney Disease Improving Global Outcomes (KDIGO)12. The KDIGO AKI Workgroup definition and staging system defines AKI on the basis of serum creatinine or urine output, as an increase in serum creatinine by ≥0.3 mg/dL (≥26.5 micromole/L) within 48 hours, or an increase in serum creatinine to ≥1.5 times baseline, occurred within the prior seven days, or Urine volume <0.5 mL/kg/hour for six hours.
KDIGO defines AKI stages as, Stage 1: Increase in serum creatinine by 0.3mg/dl in 48 hours or urine output less than 0.5ml/kg/h for 6 hours. Stage 2: Rise in serum creatinine 2-2.9 times from baseline or reduction in urine output 0.5ml/kg/hr. for ≥ 12 hours and Stage 3: Rise in serum creatinine 3 times from baseline or reduction in urine output 0.3ml/kg/hr. for ≥ 24 hours or serum Creatinine above 4mg/dl or the initiation of renal replacement therapy
Data was entered and analyzed using SPSS version 20. Quantitative variables like age and serum creatinine have been analyzed as mean +/- standard deviation. Frequencies and percentages are expressed for qualitative variables like gender, acute kidney injury, and primary diagnosis.
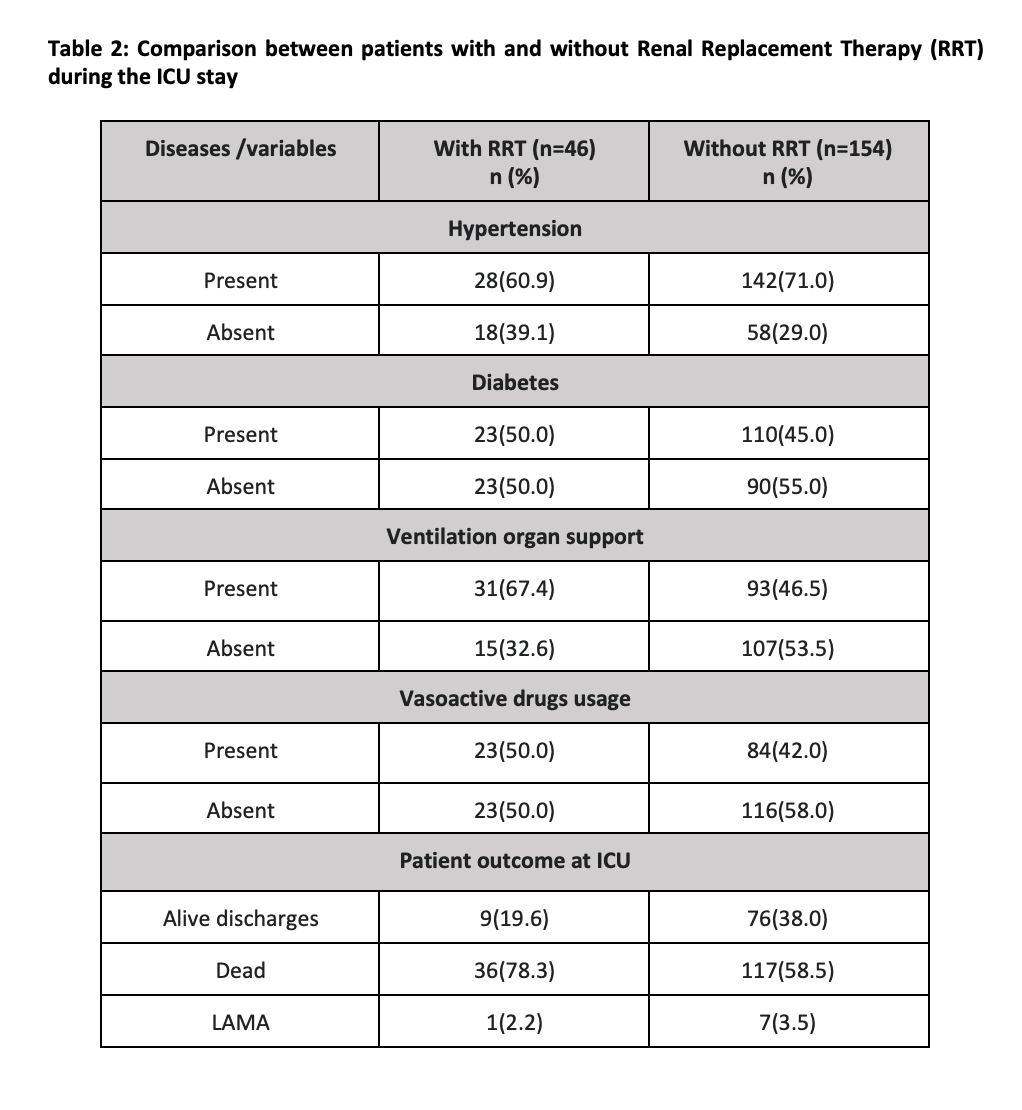
In the ICU, we had a total of 323 patients in the selected duration, admitted with COVID-19, of which 200 patients fulfilled our inclusion criteria, 70% (140) of those critically ill who developed AKI, were males with a mean age of 65.3 years (Table 1).
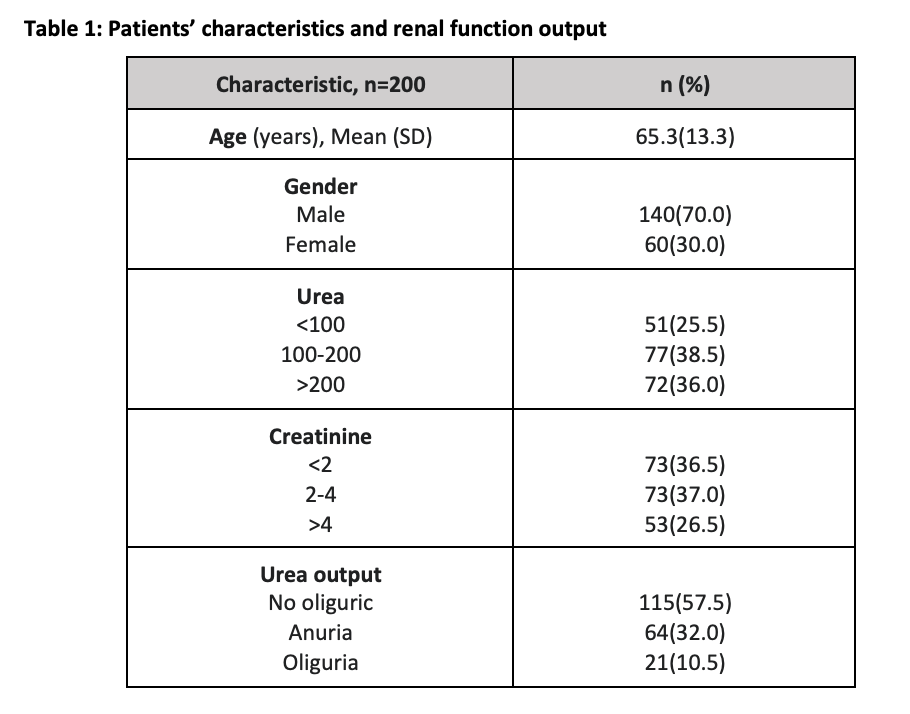
According to the KDIGO stages of renal injury, those allocated AKI stages 1, 2, and 3, respectively were 43.5%, 13.5%, and 43%. Of those, 23% (46) required RRT (figure 1). The results demonstrated that out of all 200, the overall mortality was 58.5% of patients, while 78.3% was the mortality rate of those who received RRT. Amongst the patients, 71% had a history of hypertension while almost half (45%) were affected with diabetes mellitus.
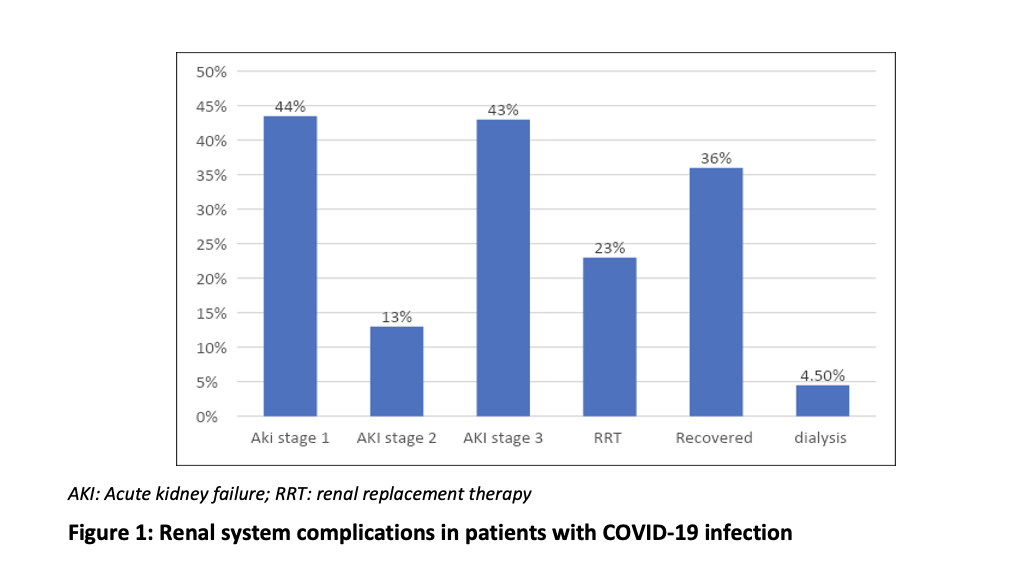
There were 9 patients (19%) who were discharged alive from those who had RRT, with 5 of these with improved and/or recovered renal status. From the RRT group, 67.4% were on ventilation organ support, and 50% required vasoactive agents. (Table 2)
This study focused on patients admitted with COVID-19 in the ICU who had a deranged renal profile at any time on or during the admission, and we established that about 43% (86) had stage-3 AKI and 23% required renal replacement therapy (Table 1). The global burden determined for AKI and COVID-19 is highly variable owing most probably to comorbidity prevalence, the sample size observed, and the availability of therapeutic options in resource-rich vs limited settings. There were initial studies advocating no relationship between COVID-19 and the development of AKI, however, since then there is ample evidence to suggest that the severity of COVID-19 pneumonia is linked to low rates of renal recovery 5,11,12. Another risk factor from a study in Spain revealed that increased Creatinine levels on admission were associated with poor prognosis13-15.
For the timeline of this study, there was a national load of 1,034,837 confirmed cases with a 2.3% death rate16 and an international of over 700,000 new cases. The frequencies we observed in this study were consistent with other centers in Pakistan with a study from Indus Hospital having a 70% prevalence of hypertension among AKI and COVID-19 patients who, in fact, were 70% predominantly males, the same as our study. Similarly, of the 31.7% who received RRT at Indus, 75.8% died as compared to our center with 23% of patients of RRT with a 78.3% mortality rate 9.
Our overall COVID-19 with AKI mortality of 58.5% in the ICU, congruent with 70% mortality at the Indus Hospital and one center in Islamabad that had a 60.5% death rate 9-11. The values mentioned above were also found to be corresponding with a study in Korea 15. Numerous data from the United States over the span of the pandemic revealed rates as high as 68% for patients developing AKI in critically ill COVID-19 patients with 34% requiring RRT, along with Hirsch et al highlighting that in patients who were on invasive mechanical ventilation (IMV), AKI was present in 90% of patients as compared to non-IMV and AKI rate of 22% 17,18. Furthermore, an audit established across the UK with over 6000 subjects demonstrated 24% of patients needing RRT, of those over 90% were on IMV or ECMO, with a mortality rate of 71% in 2020 the current mortality rate with these specifics is 62.3% 19. Our data showed that of those with AKI regardless of the stages, 46.5% of the proportion were on ventilation organ support, while in those who needed RRT, up to 67% were mechanically ventilated with over 50% on vasoactive therapy signifying severe COVID disease inclusive of MODS and AKI.
Nevertheless, there is evidence of the highly variable incidence of AKI in COVID-19; a study in Iran reported the incidence of stage-3 AKI as 17%, with a mortality rate of 47% in patients with COVID-19 and AKI 20. Another single-center study in Bahrain demonstrated an incidence of stage-3 AKI at 13.7% with only 9.3% reaching the need for RRT 21. This is in comparison to 43% of patients of this cohort of patients in stage-3 AKI with or without RRT.
There were a few limitations to our study; this is a single-center study with a limited sample size. The classification of AKI is based on the EPI eGFR calculator and is not a strictly accurate representation of the patient’s glomerular filtration rate (GFR) status. Furthermore, it is merely an observational study, therefore, causality cannot be assessed.
AKI has been associated with a worsened disease process and a higher mortality rate in critically ill patients of COVID-19. An integrated approach toward recognizing and optimizing renal protective therapy in patients with COVID-19 is required in future endeavors.
I would like to express my deep gratitude to Dilanthi Gamage for her support and encouragement.
All authors confirm that there is no conflict of interest.
This study was approved by the Ethical Review Committee of Dr. Ziauddin University Hospital, reference number 2670920KZNEP
KZ gave the main concept, developed the study design, did final editing, and reviewed the conclusions critically for drawing inferences. She is responsible for the accuracy of the data and the final version of the study. SS, MG, and AK performed data collection, data curation, and compilation of results. AS performed the data analysis and compilation of results.
- WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2021 Nov 11]. Available from: https://covid19.who.int
- Alenezi FK, Al Meshari MA, Mahida R, Bangash MN, Thickett DR, Patel JM. Incidence and risk factors of acute kidney injury in COVID-19 patients with and without acute respiratory distress syndrome (ARDS) during the first wave of COVID-19: a systematic review and Meta-Analysis. Renal Failure. 2021 Jan 1;43(1):1621-1633. Published online 2021 Dec 9. doi: 10.1080/0886022X.2021.2011747
- Park BD, Faubel S. Acute kidney injury and acute respiratory distress syndrome. Critical Care Clinics. 2021 Oct 1;37(4):835-849. Published online 2021 May 27. doi: 10.1016/j.ccc.2021.05.007
- Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nature communications. 2021 May 4;12(1):2506. DOI: 10.1038/s41467-021-22781-1
- Ahmadian E, Hosseinian Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M et al. Covid‐19 and kidney injury: Pathophysiology and molecular mechanisms. Reviews in medical virology. 2021 May;31(3): e2176. DOI: 10.1002/rmv.2176
- Shao M, Li X, Liu F, Tian T, Luo J, Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: A systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacological research. 2020 Nov 1; 161:105-107. DOI: 10.1016/j.phrs.2020.105107
- Kant S, Menez SP, Hanouneh M, Fine DM, Crews DC et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC nephrology. 2020 Dec;21(1): 1-3.DOI: 10.1186/s12882-020-02112-0
- Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. Journal of the American Society of Nephrology. 2020 Jul 1;31(7):1380-3. JASN 31(7):p 1380-1383, July 2020. | DOI: 10.1681/ASN.2020040419
- Saeed F, Alam A, Memon S, Chughtai J et al. Outcome of Acute Kidney Injury in COVID-19 Patients–A Prospective Cohort at a Single Centre in Pakistan. European Journal of Clinical Medicine. 2021 Jun 24;2(3):60-65. DOI:10.24018/clinicmed.2021.2.3.61
- Nasir N, Habib K, Khanum I, Khan N et al. Clinical characteristics and outcomes of COVID-19: Experience at a major tertiary care center in Pakistan. Journal of Infection in Developing Countries. 2021;15(4):480. DOI: 10.3855/jidc.14345.
- Abbasi MS, Masud M, Sultan K et al. Acute Kidney Injury in Patients Hospitalized with COVID-19 in a Tertiary Care Hospital of Islamabad. InMed. Forum 2021 Aug (Vol. 32, No. 8, p. 81).
- Kellum JA, Lameire N, Aspelin P, Barsoum RS et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney international supplements. 2012 Mar;2(1):1-38.https://doi.org/10.1038/kisup.2012.1.
- Pei G, Zhang Z, Peng J, Liu L, Zhang C et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. Journal of the American Society of Nephrology. 2020 Jun 1;31(6): 1157-1165.DOI: 10.1681/ASN.2020030276
- Wang L, Li X, Chen H, Yan S et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. American journal of nephrology. 2020;51(5): 343-348.DOI: 10.1159/000507471.
- Portolés J, Marques M, López-Sánchez P, de Valdenebro M et al. chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrology Dialysis Transplantation. 2020 Aug;35(8):1353-1361. DOI: 10.1093/ndt/gfaa189.
- COVID-19 Live Dashboard – Pakistan. Google Data Studio. Available from:http://datastudio.google.com/reporting/1PLVi5amcc_R5Gh928gTE8-8r8fLXJQF/page/R24IB?feature=opengraph.
- Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney international. 2020 Jul 1;98(1): 209-218.DOI: 10.1016/j.kint.2020.05.006.
- .Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—A systematic review and meta-analysis. PloS one. 2021 Feb 11;16(2): e0246-318.DOI: 10.1371/journal.pone.0246318.
- ICNARC-Reports [Internet]. [cited 2022 Mar22]. Available from: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports
- Sabaghian T, Koomleh AA, Nassiri AA, Kharazmi AB, Khalili S. Acute Kidney Injury Outcome in COVID-19 Patients. Iranian Journal of Kidney Diseases. 2022 Jan 1;16(1). DOI: 10.52547/ijkd.6610.
- Taher A, Alalwan AA, Naser N, Alsegai O, Alaradi A. Acute kidney injury in COVID-19 pneumonia: a single-center experience in Bahrain. Cureus. 2020 Aug 12;12(8). DOI: 10.7759/cureus.9693.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
