By Yawar Abbas 1, Mustajab Zehra 2, Asad Jafri3
- Assistant Professor, Department of Pathology, United Medical and Dental College-Creek General Hospital, Karachi, Pakistan
- Department of Radiology, Abbasi Shaheed Hospital, Karachi, Pakistan
- Department of Histopathology, Liaquat National Hospital, Karachi, Pakistan
DOI: https://doi.org/10.36283/PJMD12-3/003
How to cite: Abbas Y, Zehra M, Jafri A. Association of Cervical Lymph Node Metastasis and Perineural Invasion in Patients with Oral Cavity Squamous Cell Carcinoma. Pak J Med Dent. 2023;12(3): 9-15. doi: 10.36283/PJMD12-3/003
Background: Cervical lymph node metastasis (CLNM) is one of the key prognostic factors in oral squamous cell carcinoma and positive perineural invasion (PNI) is a predictor of cervical lymph node metastasis, recurrence, and decline-free survival. This study determines perineural invasion association with cervical lymph node metastasis in oral cavity squamous cell carcinoma (OCSCC).
Method: We analyzed the laboratory data collected from the surgical pathology files of a tertiary care hospital in Karachi. This retrospective study included all diagnosed cases of OCSCC that underwent cervical lymph node dissection during the 10-year period from June 2008 to March 2019. The cases were characterized according to age, gender, perineural invasion, and lymph node metastasis. The data was analyzed using SPSS and Chi-Square test was used to find the association between the cervical lymph node metastatic and PNI status.
Results: A total of 527 diagnosed cases of OCSCC with cervical neck dissection were found. It is evident from our study that OCSCC with neck dissection specimens is frequently seen in males, mostly belonging to the middle adult age group followed by young adults. Of the 527 cases, 165 showed PNI (31.3%), and 20% of the PNI cases were positive for cervical lymph node metastasis, while 40% showed benign cervical lymph nodes with negative PNI.
Conclusion: This study highlights the single parameter of PNI and its association with cervical lymph node metastasis and showed a significant relationship between perineural invasion and cervical lymph node metastatic status (p < .001).
Keywords: Perineural Invasion, Cervical Lymph Node Neck Dissection, Oral Cavity Squamous Cell Carcinoma, Lymph Vascular Invasion.
Squamous cell carcinoma of the oral cavity is a significant public health concern worldwide¹. Although it accounts for less than 5% of all cancers in Europe and Australasia, there is a notable high incidence in the Asian region. India, for instance, experiences a substantial burden of oral cavity squamous cell carcinoma, with approximately 45% of cases reported ² and presented as the most prevalent types of head and neck cancer followed by larynx and hypopharynx cancers ³, ⁴. Additionally, Pakistan also faces a considerable impact of this disease and oral squamous cell carcinoma (OSCC) has the highest prevalence in head and neck cancers and is the first and second most common cancer in males and females of Pakistan respectively.
The etiology of oral cavity squamous cell carcinoma differs between developed and developing countries. In developed nations, tobacco smoking and alcohol consumption contribute to more than 70% of oral cancer cases⁵. On the other hand, developing countries show a higher prevalence of risk factors such as betel quid chewing and tobacco use ⁶, ⁷. Furthermore, the number of new oral cancer cases is projected to rise annually, particularly in southern Asian countries with the highest incidence rates⁸. The age of onset for oral cavity squamous cell carcinoma is typically observed in the fifth and sixth decades of life, with males being more commonly affected⁹. Lymph node metastasis occurs in approximately 40% of patients with oral cancer, and the presence of cervical lymph node metastasis is a crucial parameter associated with a significant decrease in the survival rate by more than 50% ¹⁰, ¹¹. Thus, identifying individuals at risk for cervical lymph node metastasis becomes imperative.
Several parameters can influence the rate of cervical metastasis in oral cavity cancer, including the size of the primary tumor, location, T stage, grade, depth of invasion, perineural invasion, and patient compliance ¹², ¹³. The status of cervical lymph node metastasis is a vital determinant for treatment planning and prognosis. Furthermore, it is essential to recognize that CLNM can substantially impact the quality of life of affected individuals ¹⁴. The topic of PNI and lymphovascular invasion (LVI) has been frequently explored in studies concerning oral squamous cell carcinoma ¹⁵. Addressing the challenges associated with oral cavity squamous cell carcinoma, including the increasing incidence, identification of risk factors, and understanding the parameters influencing cervical lymph node metastasis, is crucial for effective management and improved outcomes for patients. In the current study, we aim to find the cervical lymph node status association between perineural invasion in patients with oral cavity squamous cell carcinoma.
Data collected from the histopathology laboratory data files of Liaquat National Hospital Karachi was analyzed. Our retrospective study included all diagnosed cases of OCSqCC that underwent cervical lymph node dissection during the period 2008-2019 (10 years and 9 months). The Inclusion Criteria were patients of either sex with different age groups: Adolescent (12 – 18 years), Young adult (19-45 years), Middle-aged (46-64 years), and Elderly (65 years or older). Tumor sites other than the oral cavity were excluded, and malignancies other than squamous cell carcinoma (such as lymphoma and salivary gland origin, etc.) were excluded. The data was analyzed using SPSS version 26. Categorical variables such as age and gender were summarized as percentages. The chi-Square test was used to evaluate the statistically significant association between the cervical lymph node metastatic status and PNI status.
The study comprised a total of 154 patients who met the inclusion requirements. The patients Based on our analysis of 527 study subjects, we found that the majority of cases fell into the middle-adult age group, followed by the young-adult age group. There was also an eminent male predilection, as a total of 414 cases were male and 113 were female. The adolescent group had the least number of cases affected by oral cavity squamous cell carcinoma. (Table 1.)
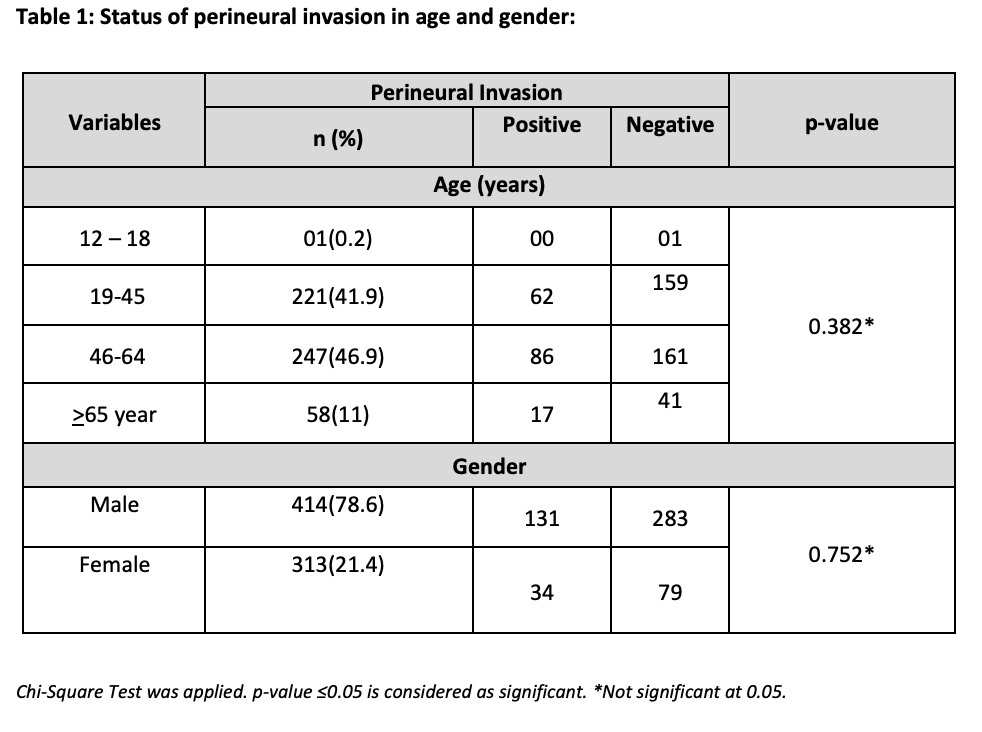
In this study, a significant male predominance (78.6%) was observed in oral cavity squamous cell carcinoma, consistent with existing literature. Throughout our 10-year 9-month study, the majority of OCSCC patients (68.69%) did not show perineural invasion (PNI). However, PNI invasion was observed in 31.3% of cases. Negative PNI status was most commonly found in the middle age group, followed by the young adult group, as indicated in Table II.
Males are more predominant in the middle and young age groups. Additionally, we have observed male gender dominance in the elderly age group (Figure. 1). However, no significant difference between genders has been noted in this age group.
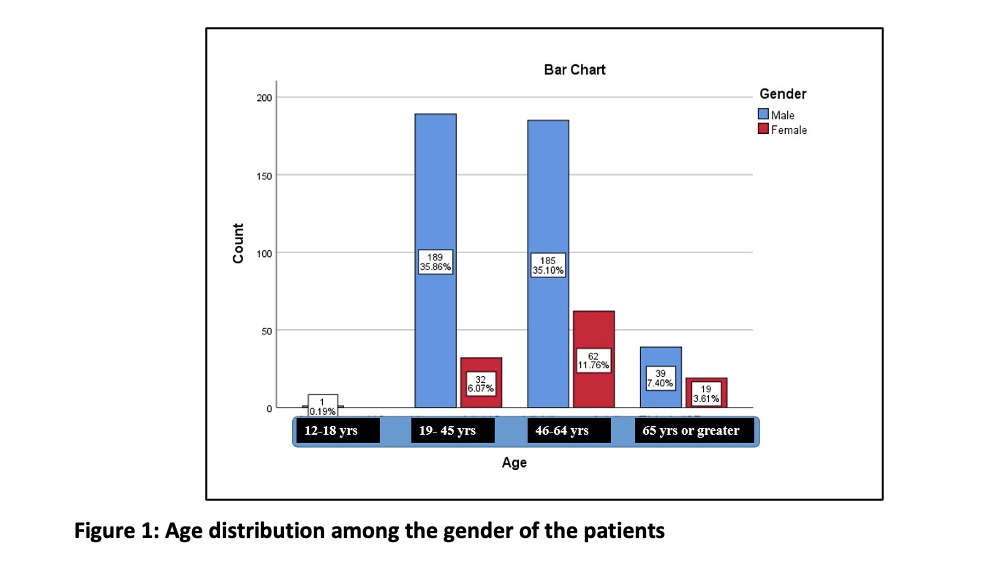
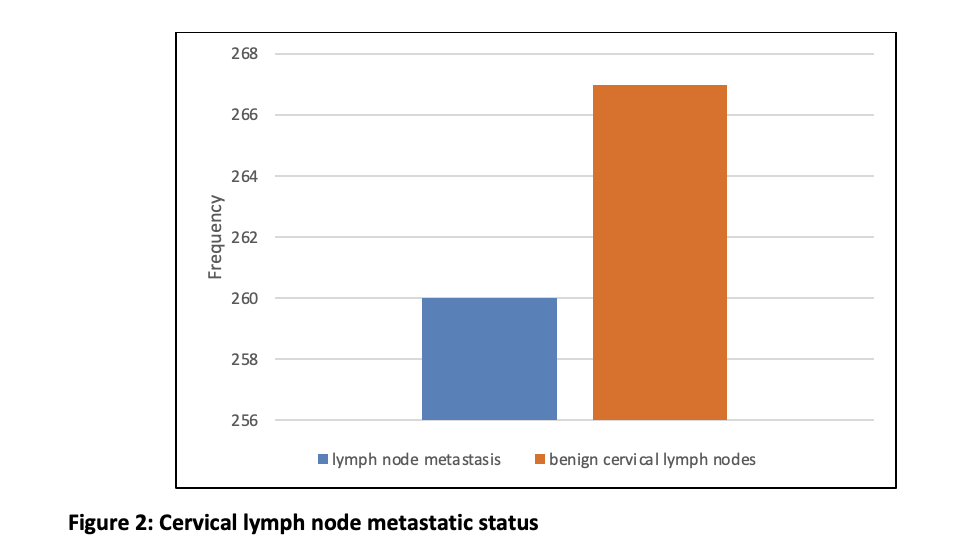
Out of the 527 cases, lymph node metastasis was seen in 260 patients, while 267 patients had benign cervical lymph nodes (Figure 2). The elderly age group was found to have a higher prevalence of cervical lymph node metastasis compared to the benign lymph node age group (Figure 3).
The comparison of frequencies of CLNM and PNI revealed that out of the total of 527 cases, 260 positive cervical lymph node metastasis cases were associated with positive PNI, representing 20.7% of the total cases. The cases were positive for cervical lymph metastasis with a negative PNI is about 28%. In contrast, there is a significant association seen with negative PNI status with benign cervical lymph nodes, about 40.4%. The Chi-Square test is significant in association with PNI status and CLNM status, with p < .001 (Table 2).
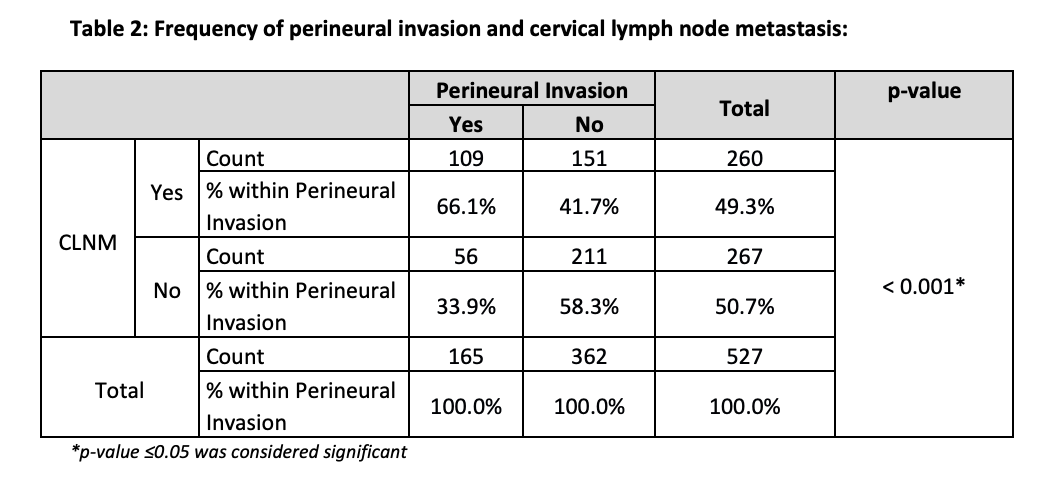
This study specifically correlated CLNM with a single parameter “PNI”. It showed that 20.7% of the patients with positive PNI in the excisional biopsy of OCSqCC had positive CLNM, in contrast to 40% of patients with negative PNI observed in benign neck nodes. There was a 10.63% incidence of positive PNI in benign lymph nodes. It was also noted that only the elderly age group showed an increased number of CLNM compared to benign lymph nodes of the same age group.
Upon conducting a comprehensive review of international literature, it became apparent that the majority of research in this area has been carried out in developed nations. For instance, an Australian study discovered no statistically significant association between perineural invasion and lymph node metastasis¹⁶. On the other hand, a study conducted in the United States demonstrated a significant association between perineural invasion and lymph node metastasis¹⁷. Additionally, a study carried out in India from 2014 to 2015 revealed a strong correlation between lymphovascular invasion (LVI) and perineural invasion with nodal metastasis¹⁸, although our study excluded the evaluation of LVI status. In a study conducted in the United Kingdom from 1989 to 1992, it was discovered that both LVI and perineural invasion played crucial roles as predictors of cervical lymph node metastasis in oral cavity squamous cell carcinoma, specifically in the mouth floor region¹⁹. Another study from the University of Michigan in the United States, which included a total of 88 cases of oral cavity squamous cell carcinoma, demonstrated that perineural invasion independently served as an adverse factor for nodal metastasis and extra-capsular spread²⁰. Similarly, an Indian study that examined both primary and recurrent tumors found a perineural invasion rate of 42.50% and 40.50%, with 34 out of 69 cases exhibiting clinically positive neck nodes²¹.
In contrast, this study focused solely on primary cases and reported a positive perineural invasion rate of 20.7% in association with positive cervical metastatic lymph nodes. Furthermore, a study from Taiwan revealed that perineural invasion independently predicted cervical lymph node metastasis²². A study from Japan highlighted the association between perineural invasion and the differentiation of neoplastic cells, nodal metastasis, and depth of invasion²³. Similarly, an Indian study identified histopathologic parameters, such as lymphovascular invasion and perineural invasion, as statistically significant indicators of positive occult nodal metastases²⁴.In another study from the United Kingdom, among 280 cases where neck-level removal was performed, 58% of patients with perineural involvement and 80% with lymphovascular invasion exhibited nodal metastasis²⁵. A study conducted in Brazil revealed that neural and vascular invasion, along with tumor thickness, were statistically correlated with lymph node metastasis²⁶. However, a study conducted in Wuhan, China, found that factors such as sex, age, lesion site, tumor stage, perineural invasion, and lymphovascular invasion did not significantly impact cervical lymph node metastasis²⁷. Furthermore, a small case study involving 140 patients with oral cavity squamous cell carcinoma conducted in Pakistan demonstrated a strong association between perineural invasion and nodal metastasis²⁸. However, a different study conducted in Pakistan did not discover a significant association with perineural invasion²⁹. Whereas, a study³⁰ showed a statistically significant correlation between PNI and lymph node involvement.
It is important to note that this particular study was conducted at a single center and excluded small biopsies obtained during the study period for oral cavity squamous cell carcinoma Furthermore, large-scale studies can also help to identify potential biomarkers or genetic signatures that can be used to predict the risk of CLNM in OCSCC, to develop more effective and personalized treatment strategies for patients with this disease.
Perineural invasion (PNI) in oral cavity squamous cell carcinoma (OCSCC) has significant prognostic implications and influences treatment decisions. The presence of PNI indicates the need for complete tumor resection and adjuvant therapy. Conversely, the absence of PNI suggests a lower risk of CLNM and may help avoid unnecessary cervical lymph node dissection. However, accurately assessing these factors can be challenging, and further research is needed to develop more reliable methods for evaluating the risk of CLNM in OCSCC.
I would like to express my deep gratitude to my supervisor “Professor Dr. Naveen Faridi”, for their guidance, enthusiastic encouragement, and useful critiques of this research work. I would also like to extend my thanks to the technicians and staff of the laboratory of the Histopathology department for their help.
All authors confirm that there is no conflict of interest.
This study has been approved by the ethics review committee of the hospital.
Data was collected from the histopathological files.
YA International literature review, Conceptualization, Laboratory. Analysis of Data done on SPSS 26, Drafting, review of histological slides. MZ Methodology, Application of statistical, mathematical review, re-analyzed study data. AJ Statistical, mathematical review, re-analyzed study data, review of cases.
- Warnakulasuriya, S. Causes of oral cancer – An appraisal of controversies. British dental journal. 2009;207(10):471-475. doi: 10.1038/sj.bdj.2009.1009
- Landis, S.H., Murray, T., Bolden, S., Wingo, P.A. Cancer statistics, 1999. CA: A cancer Journal for Clinicians. 1999;49(1):8-31. doi: 10.3322/canjclin.49.1.8
- Oral cancer statistics in India on the basis of first report of 29 population-based cancer registries. J Oral Maxillofac Pathol. 2018;22(1):18-26. doi: 10.4103/jomfp.JOMFP_113_17
- A Study of Head and Neck Cancer Patients with Reference to Tobacco Use, Gender, and Subsite Distribution. South Asian J Cancer. 2022;11(1):46-51. doi: 10.1055/s-0041-1740601
- Tuyns, A.J., Esteve, J., Raymond, L., et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case‐control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). International journal of cancer. 1988;41(4):483-491. doi: 10.1002/ijc.2910410403
- International Agency for Research on Cancer. A review of human carcinogens: personal habits and indoor combustions (Vol. 100). World Health Organization. 2012.
- Petti, S. Lifestyle risk factors for oral cancer. Oral oncology. 2009;45(4-5):340-350. doi: 10.1016/j.oraloncology.2008.05.018
- Ferlay, J., Shin, H.R., Bray, F., et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893-2917. doi: 10.1002/ijc.25516
- Müller, S. Update from the 4th Edition of the World Health Organization of Head and Neck Tumours: Tumours of the Oral Cavity and Mobile Tongue. Update from the 4th edition of the World Health Organization of head aHead and neck pathology. 2017;11(1):33-40. doi: 10.1007/s12105-017-0792-3
- Lea, J., Bachar, G., Sawka, A.M., et al. Metastases to level IIb in squamous cell carcinoma of the oral cavity: a systematic review and meta‐analysis. Head & Neck: Journal for the Sciences and Specialties of the Head and Neck. 2010;32(2):184-190. doi: 10.1002/hed.21163
- Woolgar, J.A., Triantafyllou, A., Lewis, J.S., et al. Prognostic biological features in neck dissection specimens. European Archives of Oto-Rhino-Laryngology. 2013;270(5):1581-1592. doi: 10.1007/s
- Jalisi S. Management of the clinically negative neck in early squamous cell carcinoma of the oral cavity. Otolaryngologic Clinics of North America. 2005;38(1):37-46. doi: 10.1016/j.otc.2004.09.002
- McGuirt WF, Johnson JT, Myers EN, Rothfield R, Wagner R. Floor of mouth carcinoma: the management of the clinically negative neck. Archives of Otolaryngology–Head & Neck Surgery. 1995;121(3):278-282. doi: 10.1001/archotol.1995.01890030020004
- Amaral TMP, da Silva Freire AR, Carvalho AL, Pinto CAL, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncology. 2004;40(8):780-786. doi: 10.1016/j.oraloncology.2003.10.009
- Prognostic value of lymphovascular and perineural invasion in squamous cell carcinoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133(2):207-215. doi: 10.1016/j.oooo.2021.08.02
- Wallwork BD, Anderson SR, Coman WB. Squamous cell carcinoma of the floor of the mouth: tumour thickness and the rate of cervical metastasis. ANZ Journal of Surgery. 2007;77(9):761-764. doi: 10.1111/j.1445-2197.2007. 04219.x
- Fagan JJ, Collins B, Barnes L, D’Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Archives of Otolaryngology–Head & Neck Surgery. 1998;124(6):637-640. doi: 10.1001/archotol.124.6.637
- Viswanatha SC, Hedne N, Hasan S. Correlation between histological grading, LVI and PNI of carcinoma oral tongue to lymph node metastasis. Int J Otorhinolaryngol Head Neck Surg. 2019;5(1):2018. doi: 10.18203/issn.2454-5929.ijohns20185306
- Woolgar J. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncology. 2006;42(3):229-239. doi: 10.1016/j.oraloncology.2005.05.008
- Chinn SB, Spector ME, Bellile EL, McHugh JB, Gernon TJ, Bradford CR, Wolf GT, Eisbruch A, Chepeha DB. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngology–Head and Neck Surgery. 2013;149(6):893-899. doi: 10.1177/0194599813506867
- Varsha BK, Radhika MB, Makarla S, Kuriakose MA, Kiran GS, Padmalatha GV. Perineural invasion in oral squamous cell carcinoma: Case series and review of literature. Journal of oral and maxillofacial pathology: JOMFP. 2015;19(3):335. doi: 10.4103/0973-029X.174630
- Tai SK, Li WY, Yang MH, Chu PY, Wang YF. Perineural invasion in T1 oral squamous cell carcinoma indicates the need for aggressive elective neck dissection. The American journal of surgical pathology. 2013;37(8):1164-1172. doi: 10.1097/PAS.0b013e318285f684
- Rahima B, Shingaki S, Nagata M, Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2004;97(4):423-431. doi: 10.1016/j.tripleo.2003.10.014
- Jacob TE, Malathi N, Rajan ST, Augustine D, Manish N, Patil S. Histopathological parameters predicting occult nodal metastases in tongue carcinoma cases: an Indian perspective. J Contemp Dent Pract. 2016;17(1):70-7. doi: 10.5005/jp-journals-10024-1805
- Barrett AW, Pratt MK, Sassoon I, Bisase BS, Newman L, Tighe JV, Norris PM, Dhanda J, Gulati A. Perineural and lymphovascular invasion in squamous cell carcinoma of the tongue. Journal of Oral Pathology & Medicine. doi: 10.1111/jop.13104
- d’Alessandro AF, Pinto FR, Lin CS, Kulcsar MAV, Cernea CR, Brandão LG, Matos LLD. Oral cavity squamous cell carcinoma: factors related to occult lymph node metastasis. Brazilian Journal of Otorhinolaryngology. 2015;81(3):248-254. doi: 10.1016/j.bjorl.2015.03.004
- Li Y, Liu K, Ke Y, Zeng Y, Chen M, Li W, Liu W, Hua X, Li Z, Zhong Y, Xie C. Risk Factors Analysis of Pathologically Confirmed Cervical Lymph Nodes Metastasis in Oral Squamous Cell Carcinoma Patients with Clinically Negative Cervical Lymph Node: Results from a Cancer Center of Central China. Journal of Cancer. 2019;10(13):3062. doi: 10.7150/jca.30502
- Abidi F, Hosein M, Butt SA, Zaidi AB, Anjum A, Fatima S. Association of Clinicopathological Features with Lymph Node Metastasis: A Cross-Sectional Study of Oral Squamous Cell Carcinoma Patients. Journal of Advances in Medicine and Medical Research. 2020;32(2):46-53. doi: 10.9734/jammr/2020/v32i230365
- Mirza S, Hadi NI, Akram S, Wahab N, Akram Z. Histopathological predictors of nodal metastasis in oral squamous cell carcinoma. Pak J Med Dent 2016;5(3):12-16
- Jangir NK, Singh A, Jain P, Khemka S. The predictive value of depth of invasion and tumor size on risk of neck node metastasis in squamous cell carcinoma of the oral cavity: A prospective study. Journal of Cancer Research and Therapeutics. 2022 Jul 1;18(4):977-83.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
