By Faridah Amin1, Saima Akhter2, Areeba Abdullah3, Noureen Durrani4, Saleha Shehzad5, Kiran Akhtar6
AFFLIATIONS:
- Director, College of Family Medicine and Public Health, The Indus Hospital, Karachi, Pakistan.
- Department of Chest Medicine, Liaquat National Hospital and Medical College, Karachi, Pakistan.
- Medical Student, Liaquat National Hospital and Medical College, Karachi, Pakistan.
- Department of Publication, Liaquat National Hospital and Medical College, Karachi, Pakistan.
- Department of Radiology and Imaging, Liaquat National Hospital and Medical College, Karachi, Pakistan.
- Outpatient Department Services and Radiology, Liaquat National Hospital and Medical College, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-4/006
ORCID iD: 0000-0002-0937-2076
How to cite: Amin F, Akhter S, Abdullah A, Durrani N, Shehzad S, Akhtar K. Efficacy of Sinopharm Vaccine Among Healthcare Workers of Pakistan. Pak J Med Dent. 2022;11(4): 34-40. doi: 10.36283/PJMD11-4/006
Background: The advent of a vaccine for COVID-19 has been a breakthrough to prevent infection from the virus. The objective of this study was to compare the frequency and severity of COVID-19 infection among vaccinated and non-vaccinated healthcare workers (HCWs).
Methods: This was a prospective cohort study conducted on HCWs at Liaquat National Hospital, Karachi. The participants n=2500 were recruited before (November 2020 to January 2021) and after vaccination (February 2021 to May 2021). A detailed history including vaccination status, from the patients, was taken by treating physicians. All recruited patients were followed up for three months for upper respiratory tract symptoms. Chi-square test/Fisher exact was applied for associations. Poisson regression was applied to compute the incidence rate. A p-value ≤0.05 was defined as statistically significant.
Results: The 2500 participants were included in each group of this cohort study. The median age of unvaccinated and vaccinated healthcare workers was 33 (27 – 42.5) years and 35(29 – 45) years respectively. During three months, 166 (6.64%) workers acquired COVID-19 infection yielding an incidence of 7.6 per 10,000 person-days. All the cases were mild and were managed at their home during their quarantine period. Within 3 weeks after the first dose, 2(0.08%) workers had COVID-19 infection with an incidence rate of 0.4 per 10,000 person-days. After administration of the second dose, 4(0.16%) found COVID-19 positive within 21 days with an incidence of 0.8 per 10,000 person-days.
Conclusion: Vaccine provides significant (p<0.001) protection against COVID-19 infection in healthcare workers in a highly infective environment.
Keywords: Health Care Worker; Immunization; Vaccination; COVID-19.
At the start of February 2021, Pakistan launched a nationwide immunization program against COVID-19 1. The priority was given to healthcare workers (HCWs) from the beginning of the pandemic as they were directly exposed. The advent of a vaccine for COVID-19 has been a breakthrough to prevent infection from the deadly virus. Different vaccinations have been offered by different countries to their populations, all of which have shown reasonable efficacy and adverse effect profile. The efficacy of the Chinese vaccine has been recently reported as 50%, while a 65% efficacy was reported from Indonesia and Pakistan in the general population2,3.
Health care workers (HCWs) have a critical element in teaching the overall public approximately the supply of the vaccine and its implications withinside the coming years3. In Pakistan, HCWs are being prioritized for an early primarily Chinese-based COVID-19 vaccination program4. This is being mandated at some point in the West, prioritizing high-hazard groups, and HCWs being diagnosed as such. Therefore, it is far critical to recall HCW attitudes in the direction of the COVID-19 vaccine because it will cause higher dissemination of expertise amongst the overall public. Given the paucity of statistics concerning vaccines in South-East Asia among HCWs, the study was carried out to mark the significance of the Sinopharm vaccine among HCWs.
Literature is available regarding vaccine effectiveness among healthcare workers3-5. Studies determining the incidence of post-vaccine among healthcare workers are limited in Pakistan6,7. Therefore, the study aimed to compare the frequency of COVID-19 infection among vaccinated and non-vaccinated healthcare workers regarding Sinopharm administration.
This prospective cohort study was conducted in Liaquat National Hospital, Karachi. Approval was taken from the institutional ethical review committee. Healthcare workers from November 2020 to January 2021 were unvaccinated while healthcare workers from February 2021 to May 2021 were vaccinated. All employees were recruited and followed till 12 weeks after receiving the second dose of vaccination. Healthcare workers who resigned and died due to another cause except for COVID-19 infection within 3 months of study duration were excluded from the study.
HCWs were instructed to report symptoms they experienced during this period. The results of COVID-19 PCR were documented and the HCW was followed up for clinical outcome. Vaccine effectiveness was assessed on the following 4 parameters; decrease in overall incidence in the study population, the need for COVID-19 hospitalization, decrease in disease severity, oxygen requirement and decrease in mortality after vaccination.
Another study reported that the COVID-19 positive rate was 52% and 48% among unvaccinated and vaccinated healthcare workers respectively8. Taking a 95% confidence interval and 80% power, a sample of 2452 healthcare workers per group was required. Data were analyzed using Stata version 16. Frequencies/percentages were computed to summarize categorical variables. Numerical variables were expressed as median with an interquartile range after assessing the normality assumption. Chi-square test/Fisher exact was applied. Poisson regression was applied to compute the incidence rate. Effects of covariates were adjusted to calculate adjusted incidence. The exposure command was used to account for personal time in the model. A p-value ≤0.05 was defined as statistically significant.
A total of n=2500 participants were included in each cohort. The median age of unvaccinated and vaccinated healthcare workers was 33 (27 – 42.5) years and 35(29 – 45) years respectively. Age groups were significantly different among the unvaccinated and vaccinated groups (Table 1, Figure 1 depicts gender distribution among the two groups which were significantly different (p<0.001). Risk exposure is displayed in Figure 2 among the two groups. The distribution of risk exposure was significant among the group (p<0.001).
Table 1: Comparison among unvaccinated and vaccinated healthcare workers.
| Age in groups | Unvaccinated
Frequency n (%) |
Vaccinated
Frequency n (%) |
p-Value |
| Young
(18-39 years) |
1680 (67.2) | 1519 (60.8) | **<0.001 |
| Middle
(40-59 years) |
709 (28.4) | 847 (33.9) | |
| Elderly
(60 years and above) |
111 (4.4) | 134 (5.4) |
**Significant at p<0.01.
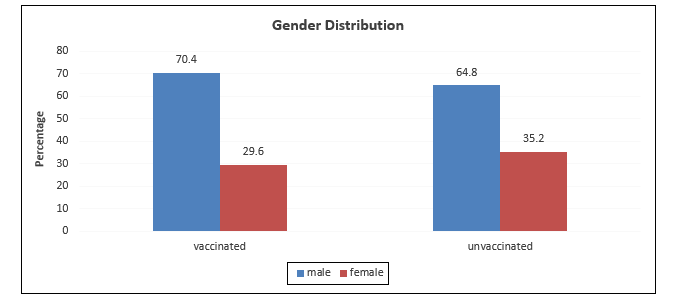
Figure 1: Gender distribution among vaccinated and unvaccinated groups.
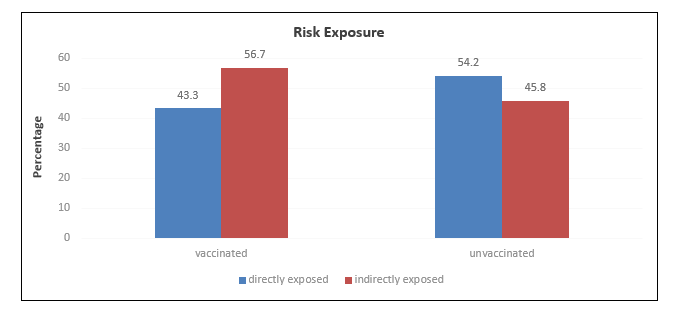
Figure 2: Distribution of risk exposure among vaccinated and unvaccinated groups.
During three months, out of 2500 unvaccinated healthcare workers, 6.64% of workers acquired COVID-19 infection. Among the unvaccinated group, 84.9% were symptomatic COVID-19 cases with the most frequent symptom of fever (70.9%) followed by cough (66%), muscle ache (53.2%), sore throat (37.6%) and shortness of breath (9.2%). All the cases were mild and did not require hospital admission and were managed at their home during their quarantine period. The median days to symptom resolution were 7(3 – 10) days. Out of 166 (64%), 15(9%) developed hypoxia during their quarantine period. The incidence rate of COVID-19 infection among unvaccinated people is presented in Table 2.
Within 3 weeks of the first dose, 2(0.08%) workers came out to be COVID-19 infected. Both were female, symptomatic and directly involved in patient care. One of them had mild symptoms of cough and sore throat and the other had symptoms of shortness of breath and muscle ache. Symptoms of both were resolved within a week. Table 2 represents the incidence rate of COVID-19 infection within 21 days of the first vaccine dose. The incidence rate among sub-groups of other study variables is also represented in Table 2.
Table 2: Incidence rate of COVID-19 among healthcare workers.
| Follow-up | Unvaccinated | Vaccinated | |||
| Within 3 months | Within 21 days after dose 1 | Within 21 days after dose 2 | Within 42 days after dose 2 | Within 84 days after dose 2 | |
| Frequency (n) | 2500 | 2500 | 2500 | 2500 | 2381 |
| Events (n) | 166 | 2 | 4 | 6 | 23 |
| Person-days | 217307 | 52483 | 52458 | 104860 | 199406 |
| Incidence rate 10,000 person-days |
7.6 | 0.4 | 0.8 | 0.6 | 1.2 |
| Age in groups | |||||
| Young age (18-39 years) |
8.7 | 0.3 | 1.3 | 0.8 | 1.3 |
| Middle age (40-59 years) |
6.0 | 0.6 | 0.0 | 0.3 | 0.2 |
| Elderly (60 years and above) |
7.3 | 0.0 | 0 | 0 | 1.6 |
| Gender# | |||||
| Male | 7.0 | 0.0 | 1.1 | 0.7 | 0.9 |
| Female | 8.8 | 1.3 | 0.0 | 0.3 | 1.7 |
| Risk Exposure# | |||||
| Directly exposed | 8.3 | 0.9 | 1.3 | 0.9 | 2.1 |
| Indirectly exposed | 6.9 | 0.0 | 0.3 | 0.3 | 0.4 |
#The incidence rate is expressed as 10,000 person-days.
Crude incidence rate of COVID-19 infection was 99% lower in the vaccinated group than in the unvaccinated group. The incidence rate was also significantly lower in the vaccinated group as compared to the unvaccinated when effects of age, gender, risk exposure and person-days were adjusted (Table 3).
After administration of the second dose, 4(0.16%) were found to be COVID-19 positive within 21 days and all were males and young. 3 of them were directly involved in patient care and only one case was asymptomatic. One patient had only symptoms of fever which were resolved in three days. One had symptoms of fever, muscle ache and cough and the symptoms were resolved within a week. One case presented with symptoms of fever, sore throat, muscle ache and flu and within 5 days symptoms were resolved. None of them required hospital care nor developed hypoxia. The crude incidence rate and adjusted incidence rate for COVID-19 were significantly lower in the vaccinated group than in the unvaccinated group (Table 3).
Only 6(0.24%) were infected with COVID-19 within 6 weeks of administering the second dose of vaccine. 5 of them were belonging to a younger age group, male and symptomatic COVID-19 case. 2 of these 6, were not directly involved in patient care. Presenting symptoms were fever (83.3%), cough (50%), muscle ache (50%), sore throat (33.3%) and flu (16.7%) and symptoms were resolved within 7 days of onset. Hospital admission was not required and there was no reported hypoxia among these 6 infected workers. Adjusted COIVD-19 incidence was 92% lower in vaccinated people than unvaccinated group (Table 3).
Table 3: Adjusted incidence rate ratios for COVID-19 infection.
|
Variables |
First dose | Second dose | ||||||
| 3 weeks | 3 weeks | 6 weeks | 12 weeks | |||||
| aIRR
(95% CI) |
p-Value | aIRR
(95% CI) |
p-Value | aIRR
(95% CI) |
p-Value | aIRR
(95% CI) |
p-Value | |
| Age groups | ||||||||
| Young
(18-39 years) |
Ref | Ref | Ref | Ref | ||||
| Middle
(40-59 years) |
0.73
(0.51 – 1.06) |
0.100 | 0.69
(0.47 – 1) |
0.050 | 0.71
(0.48 – 1.01) |
0.058 | 0.69
(0.48 – 1) |
*0.047 |
| Elderly
(60 years and above) |
0.37
(0.12 – 1.18) |
0.084 | 0.35
(0.11 – 1.10) |
0.073 | 0.35
(0.11 – 1.10)
|
0.070 | 0.70
(0.35 – 1.39) |
0.309 |
| Gender | ||||||||
| Male | Ref | Ref | Ref | Ref | ||||
| Female | 1.20
(0.87 – 1.65) |
0.278 | 1.11
(0.80 – 1.53) |
0.539 | 1.12
(0.81 – 1.54) |
0.517 | 1.17
(0.86 – 1.59) |
0.309 |
| Worker | ||||||||
| First line | Ref | Ref | Ref | Ref | ||||
| Second line | 0.91
(0.66 – 1.26) |
0.573 | 0.90
(0.65 – 1.24) |
0.517 | 0.90
(0.65 – 1.24) |
0.512 | 0.80
(0.59 – 1.09) |
0.154 |
| Group | ||||||||
| Vaccinated | 0.05
(0.01 – 0.21) |
**<0.001 | 0.10
(0.04 – 0.28) |
**<0.001 |
0.08
(0.03 – 0.18) |
**<0.001 | 0.16
(0.1 – 0.25) |
**<0.001 |
| Unvaccinated | Ref | Ref | Ref | Ref | ||||
aIRR= Adjusted incidence rate ratio, CI= Confidence interval, Ref= Reference category, **Significant at p<0.01.
Within 12 weeks of injecting the second dose, 23(0.92%) employees came out to be COVID-19 positive. Most of them were males (56.5%) and directly related to patient care (78.3%). 69.6% and 30.4% were young and middle-aged respectively. 17.4% of cases were asymptomatic. Among symptomatic patients, presenting symptoms included fever (84.2%), cough (68.4%), muscle ache (63.2%), sore throat (31.6%) and flu (5.3%). The adjusted incidence rate was significantly lower in the vaccinated group within 12 weeks were also significantly lower (3).
Since the beginning of the COVID-19 outbreak, Pakistan is reported to be ranking 3rd in the highest number of total COVID-19 cases among all countries of the eastern Mediterranean region1. COVID-19 prevalence among the population from both; developed and developing countries reveal a high rate of infection among the HCWs 9. Over the past year, a variety of medications have been utilized to treat coronavirus disease 2019 (COVID-19). Although most treatments for COVID-19 were ineffective, researchers have promoted herd immunity as a means of containing the present outbreak. A person can effectively and safely defend themselves against COVID-19 through vaccination. Even though the COVID-19 virus has been studied extensively since it was originally discovered, there are still many unanswered questions.
Most studies done previously in Pakistan focused on the general population and include people who stayed at home during isolation. As the risk of infection was highest among the healthcare workers who have been the heroes of the pandemic; throughout the world and continued working and got high exposure during their work hours, the data from this group is important to determine the actual effectiveness of the vaccine in high contact scenarios. Hence this study determines the proportion and severity of infection among the vaccinated and non-vaccinated, high-risk group of healthcare professionals working on the frontline in one of the largest tertiary care hospitals in Pakistan.
In our study, 166 COVID-19 positive HCWs were identified during the pre-vaccination period whereas only 23 cases were COVID-19 positive following 3 months post-vaccination period (after the second dose). None of the patients required hospital admission and all had mild disease. Compared to COVID-19 data from the general population, 64.2% of the cases were reported to be moderate to severe in nature9,10. In this study, there were no mortalities reported in the pre or post-vaccination period in HCWs whereas data from the general population showed a mortality rate of 20.6% in COVID-19 positive patients10. This difference in the severity of the disease and mortality may be because HCWs have quick access to hospital care and direct contact with doctors for their treatment compared to the general population.
Unvaccinated individuals have an 11 times greater risk of death from COVID-19 infection than those who are fully vaccinated11. A study in the US reported that vaccinated people were 10 times less likely to be admitted to hospital and five times less likely to be infected than unvaccinated people12. This study demonstrated significant effectiveness against COVID-19 infection, with a decrease in incidence rate from 7.6 per 10,000 person-days in the pre-vaccination period to 0.8 per 10,000 person-days following 21 days after the second dose of vaccination. This is consistent with the data gathered from another part of World2. In this study, the frequency of middle age and the elderly population was significantly higher among the vaccinated group even though this group of population is more vulnerable, a lower incidence rate was observed in the vaccinated group indicating the promised vaccine efficacy13,14. Some studies showed a decline in the effectiveness of the vaccine in the elderly population whereas our study showed decreased incidence of COVID-19 cases in all vaccinated age groups, including the elderly population11.
Not only with Sinopharm but all other vaccines showed a considerable decrease in the incidence rate of COVID-19 infection. A trial from the USA demonstrated 95% effectiveness of the Pfizer/Biotech vaccine in the general population15,16. Comparable results were seen with Moderna Vaccine17,18. Nation-based surveillance has high heterogeneity in populations with different exposure intensities to virus19,20. Though a clear effect was seen after the vaccination in clinical trials data from high-risk subgroups is of great importance21. Brazilian Double blinded RCT is being conducted where the effect of inactivating vaccine in HCW will be groundbreaking and will demonstrate the real-world effectiveness of the vaccine in high-risk group22. The severity of the disease was also decreased and symptoms were considered mild in vaccinated HCWs. Jara et al reported 87.5% prevention of hospitalization and 90.3% prevention of ICU admissions among a fully vaccinated population20.
The decrease in the incidence rate of COVID-19 infection after 1st dose is around 90% in our study, pointing out the early effect of the vaccine and is consistent with international data23,24. Hence, the impact of vaccination on our HCWs was evident even before the application of the second dose, but the data demonstrated a gradual increase in the incidence rate of COVID-19 from 0.8 per 10,000 person days at 21 days after the second dose to 1.2 per 10,000 person days at 84 days after the second dose of vaccination. The number of cases increases after 3 months of the vaccine when compared to 3 weeks, which shows a possible progressive fall of protection and need for a booster. That was also seen in other studies25.
Our study has a few limitations. Healthcare workers were first randomly divided into two groups. One group was studied for COVID-19 status in the pre-vaccination period while the other group was studied after receiving the vaccination, hence, due to this random split, the frequency of directly exposed workers was significantly higher in the unvaccinated group. Although we have paid special attention and conducted several classes for staff to perform nasal PCR inappropriate techniques can alter the estimate of vaccine effectiveness. Moreover, during the period of study, only one vaccine type was available and no comparison with other vaccines was performed.
Two doses of the Sinopharm vaccine are effective among Health Care Workers, in decreasing incidence levels and the severity of disease including hospitalization and oxygen demand.
The authors would like to acknowledge the colleagues and paramedical staff for their assistance.
The authors declared no conflict of interest.
Approval was taken from the Liaquat National Hospital ethics committee.
Informed consent was taken from the patients.
FA conceptualized the study. SA and FA designed the study protocol. AA, KA and ND were involved in data collection. AA and ND performed data analysis, result write-up and interpretation. AA, KA and SS wrote the initial draft of the study. FA and SA critically reviewed and revised the initial draft. All authors read and approved the manuscript.
- World Health Organization. COVID-19 situation updates for week 8; 2021 [cited 2022 Aug 21]. Available from: http://www.emro.who.int/pandemic-epidemic-diseases/covid-19/covid-19-situation-updates-for-week-8-2127-february-2021.html
- Baraniuk C. What do we know about China’s covid-19 vaccines? BMJ. 2021;373:1-2. doi: 10.1136/bmj.n912
- Mallapaty S. China COVID vaccine reports mixed results—what does that mean for the pandemic. Nature. 2021;15:1-6. doi: 10.1038/d41586-021-00094-z
- Amit S, Beni SA, Biber A, Grinberg A, Leshem E, Regev-Yochay G. Postvaccination COVID-19 among healthcare workers, Israel. Emerg Infect Dis. 2021; 27(4): 1220-1222. doi: 10.3201/eid2704.210016
- Pilishvili T, Gierke R, Fleming-Dutra KE, Farrar JL, Mohr NM, Talan DA, et al. Effectiveness of mRNA Covid-19 vaccine among US health care personnel. N Engl J Med. 2021;385(25):1-13. doi: 10.1056/NEJMoa2106599
- Khan JA, Satti L, Bizanjo M, Ather NA. Comparison of clinical characteristics and outcome between vaccinated and non-vaccinated patients of covid-19 during the delta variant-dominated fourth wave in a tertiary care hospital in Karachi, Pakistan. Cureus. 2022; 14(4):1-6. doi:7759/cureus.23726
- Maroof S, Bakht N, Saleem S, Nisar S, Rashid Z, Mansoor E, et al. COVID-19 vaccine breakthrough infections among health care workers in military institutes of Pakistan–till 30th June 2021. Pak Armed Forces Med J . 2021;71(4):1471-1475.
- Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875-877. doi: 10.1016/S0140-6736(21)00448-7
- Zheng C, Shao W, Chen X, Zhang X, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022; 114:252-260. doi: 1016/j.ijid.2021.11.009
- Ahsan T, Rani B, Siddiqui R, Glenis DS, Memon R, Lutfi I, et al. Clinical variants, characteristics, and outcomes among COVID-19 patients: a case series analysis at a tertiary care hospital in Karachi, Pakistan. Cureus. 2021;13(4):1-12. doi 10.7759/cureus.14761
- Dyer O. Covid-19: Unvaccinated face 11 times risk of death from delta variant, CDC data show. 2021;374: 1-2. doi: 10.1136/bmj.n2282
- Scobie HM, Johnson AG, Suthar AB, Severson R, Alden NB, Balter S, et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status—13 US jurisdictions, April 4–July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(37): 1284-1290. doi: 10.15585/mmwr.mm7037e1
- Rodriguez-Morales AJ, Franco OH. Assessing the effectiveness of COVID-19 vaccines in older people in Latin America. Lancet Healthy Longev. 2022;3(4):e219-e220. doi: 10.1016/S2666-7568(22)00073-3
- Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021; 50(2), 279-283. doi: 10.1093/ageing/afaa274
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646-1657. doi: 10.1016/S0140-6736(21)00677-2
- Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, et al. Covid-19 vaccine effectiveness in New York state. N Engl J Med. 2022;386(2):116-127. doi: 10.1056/NEJMoa2116063
- World Health Organization. The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know; [cited 2022 Aug 21]. Available from: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know
- Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study. Lancet Reg Health Am. 2022;6:1-14. doi: 10.1016/j.lana.2021.100134
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;15;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8
- A Jara, EA Undurraga, C Gonzalez, F Paredes, T Fontecilla, G Jara, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875-884. doi: 10.1056/NEJMoa2107715
- Patel MK, Bergeri I, Bresee JS, Cowling BJ, Crowcroft NS, Fahmy K, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: Summary of interim guidance of the World Health Organization. Vaccine. 2021;39(30):4013-4024. doi: 10.1016/j.vaccine.2021.05.099
- Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MT, Batista AP, Zeng G, et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac–PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):1-3. doi: 10.1186/s13063-020-04775-4
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205-211. doi: 10.1038/s41591-021-01230-y
- Chodick G, Tene L, Patalon T, Gazit S, Tov AB, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6):1-9. doi: 10.1001/jamanetworkopen.2021.15985
- Krueger KM, Halasa N, Ison, MG. SARS-CoV-2 Vaccine in dialysis patients: Time for a boost? Am J Kidney Dis. 2022; 79(2): 162-163. doi: 10.1053/j.ajkd.2021.10.003
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
