By Hamza Tariq1, Subramanya Sakaleshpura Mallikarjunappa1, Neelima Valluru1, Abdullah Almajnooni1, Waqas Mahmud1, Swathi Reddy2, Paolo Gattuso1, Lin Cheng1
AFFLIATIONS:
- Department of Pathology, Rush University Medical Center, Chicago, United States.
- Department of General Surgery, Rush University Medical Center, Chicago, United States.
DOI: https://doi.org/10.36283/PJMD11-4/002
ORCID iD: 0000-0001-8325-365X
How to cite: Tariq H, Mallikarjunappa SS, Valluru N, Almajnooni A, Mahmud W; Reddy S, et al. Cytomorphologic Features of Metastatic Sarcomas in Body Cavity Fluids Overlaps with Other Common Malignancies. Pak J Med Dent. 2022;11(4): 3-14. doi: 10.36283/PJMD11-4/002
Background: Sarcomas rarely involve body cavity fluids, accounting for less than 5% of all malignant effusions. Most existing literature describes the cytomorphologic features of sarcomas in the setting of fine-needle aspiration and/or touch preparations of targeted tissue biopsies. The objective of this study was to investigate the morphologic spectrum displayed by metastatic sarcomas in body cavity fluid specimens.
Methods: The pathology database at our institution was searched for sarcoma cases involving pleural, pericardial, and peritoneal fluids between 1994 and 2019. Their clinical presentations and cytopathologic features were reviewed. Immunohistochemical (IHC) stains were performed on the cellblock sections at our institution on the DAKO Autostainer (DAKO, Carpinteria, CA).
Results: Nineteen cases were identified, including 14 females and 5 males, ranging in age from 4 to 88 years old (median: 28.6 years). The body cavity sites were 11 pleural, 7 peritoneal, and 1 pericardial. There were 5 rhabdomyosarcomas, 4 osteosarcomas, 4 Ewing sarcomas, 2 endometrial stromal sarcomas, 2 leiomyosarcomas, 1 clear cell sarcoma, and 1 liposarcoma. Malignant pleural effusion was the first presentation in only 2 cases (both alveolar rhabdomyosarcomas). Three different cytomorphologic patterns were seen: 1-glandular cell pattern showing large epithelioid cells with vacuolated cytoplasm (3 cases), a 2-pleomorphic single-cell pattern showing large highly atypical cells (6 cases); 3-small round blue cell pattern showing small to intermediate-sized dyscohesive cells with high NC ratio (10 cases).
Conclusion: The cytomorphologic spectrum exhibited by metastatic sarcomas in body cavity fluids often overlaps with other common malignancies such as metastatic adenocarcinomas, poorly differentiated carcinomas, and lymphomas.
Keywords: Sarcoma; Cytology; Peritoneal.
Cytomorphologic analysis of pleural, pericardial, and peritoneal fluids is a routinely performed test for the diagnosis and monitoring of neoplastic and non-neoplastic disorders. In the context of neoplastic disorders, body cavity fluid analysis plays a critical role in diagnosing, staging, and monitoring disease status1. Most malignant effusions in adults are caused by metastatic tumors of epithelial origin most commonly from pulmonary, breast, gastrointestinal, and ovarian primaries2. Non-epithelial tumors such as lymphoma, leukemia, melanoma, mesothelioma, and germ cell tumors comprise a smaller proportion of cases3.
Sarcomas are neoplasms of mesenchymal origin constituting only 0.6% of all malignancies in the United States4. Hence, malignant effusions due to sarcomas are exceedingly rare and highly unusual in the practice of cytology. Most existing literature describing the cytologic features of various sarcomas is in the setting of fine-needle aspiration (FNA) of targeted lesions and touch preparations (TPs) of tissue biopsies. Morphologic features of sarcomas in liquid-based preparations of body cavity fluids are not well studied and can cause significant diagnostic difficulty. Virtually any sarcoma can metastasize to serosal surfaces; therefore, the spectrum of morphologic findings is broad and highly variable. Recognizing these entities in body cavity fluids is critical for their diagnosis and management. We undertook a retrospective study at our institution with the objective of a better understanding of the morphologic characteristics of metastatic sarcomas in body cavity fluid specimens.
The cytology database at our institution (CoPathPlus, Cerner Corporation, North Kansas City, MO) was searched for cases of metastatic sarcomas involving pleural, peritoneal, and pericardial cavities between 1994 and 2019. A chart review in EPIC (Epic Systems Corporation, Verona, WI) was performed for patient age, gender, past medical history, and clinical presentation. The time from initial diagnosis of sarcoma to body cavity involvement was calculated for patients with a preexisting history of sarcoma.
The pathologic materials were retrieved and reviewed by two pathologists. Thin Prep slides were prepared from the body cavity fluid according to the ThinPrep non-GYN test (Hologic, Marlborough, MA) protocol and stained by the Papanicolaou method. Cellient (Hologic, Marlborough, MA) cellblocks were also prepared and the sections were stained with hematoxylin and eosin (H and E). Immunohistochemical (IHC) stains were performed on the cellblock sections at our institution on the DAKO Autostainer (DAKO, Carpinteria, CA).
A total of 19 cases of sarcomas involving body cavities were identified. These include 5 rhabdomyosarcomas (4 alveolar and 1 embryonal), 4 osteosarcomas, 4 Ewing sarcomas (ES), 2 leiomyosarcomas, 2 high-grade endometrial stromal sarcomas (HG-ESS), 1 myxoid liposarcoma, and 1 clear cell sarcoma. The body cavities were pleural in 11 cases, peritoneal in 7, and pericardial in 1 case. The patients ranged in age from 4 to 88 years old (median: 28.6 years) with a male to female ratio of 0.3:1.0. All but 2 patients (17/19; 89.5%) had a pre-existing history of sarcoma. Two patients with alveolar rhabdomyosarcomas presented with malignant effusions as their first manifestation. For cases with a preexisting history, the time from the initial diagnosis of sarcoma to body cavity involvement ranged from 3 weeks to 8.9 years (median: 26.7 months). Cellblocks yielded adequate cellularity for diagnosis and IHCs in 14/19 cases. Based on the cytomorphologic findings in ThinPrep, the cases were divided into three distinct patterns:
- Glandular cell pattern characterized by large epithelioid cells with abundant vacuolated cytoplasm (3 cases).
- Pleomorphic single-cell pattern characterized by large highly atypical cells with irregular and often multilobulated nuclei (6 cases).
- Small round blue cell pattern characterized by dyscohesive small to intermediate-sized cells with a high nuclear to cytoplasmic (NC) ratio (10 cases). The clinicopathologic findings of these 19 cases are summarized in Table 1.
Table 1: Clinical and cytomorphologic features of sarcomas involving body cavity fluids.
| Case number | Age | Gender | Prior history of sarcoma (Histologic subtype, site) | Body cavity involved | Time from the initial diagnosis to body cavity involvement (months) | Cytology diagnosis | Cellblock | Results of immunohistochemistry | Cytology pattern |
| 1 | 50 | F | Myxoid liposarcoma, thigh | Pleural | 2.5 | Positive | Occasional clusters of large epithelioid cells with prominent nucleoli and abundant cytoplasm | Positive: Desmin, S100
Negative: CK5, Calretinin, AE1/AE3 |
Glandular cell pattern |
| 2 | 32 | F | Osteosarcoma, femur | Pleural | 17.5 | Suspicious | Scant clusters of large atypical cells with prominent nucleoli and a small amount of eosinophilic cytoplasm | Positive: None
Negative: AE1/AE3, MOC31, S100, D2-40, SMA, CK5 |
Glandular cell pattern |
| 3 | 75 | F | Clear cell sarcoma, index finger | Pleural | 28.6 | Positive | Crowded clusters of large epithelioid cells with abundant cytoplasm | Positive: HMB45, S-100, and vimentin
Negative: AE1/AE3, MOC31, CAM5.2 |
Glandular cell pattern |
| 4 | 38 | F | Leiomyosarcoma, uterus | Peritoneal | 3.1 | Atypical | Acellular | Not performed | Pleomorphic single-cell pattern |
| 5 | 57 | F | Leiomyosarcoma, uterus | Peritoneal | 1.1 | Positive | A few scattered bizarre cells with multilobulated nuclei and coarse chromatin and a few spindle cells | Positive: SMA, Desmin
Negative: AE1/AE3, S100, CK5, Calretinin |
Pleomorphic single-cell pattern |
| 6 | 17 | M | Osteosarcoma, femur | Pleural | 66.5 | Positive | Scant singly scattered highly atypical large cells with coarse chromatin and often multilobulated nuclei | Positive: None
Negative: CAM5.2, AE1/AE3, D2-40, CK5 |
Pleomorphic single-cell pattern |
| 7 | 24 | F | Osteosarcoma, femur | Pleural | 107.1 | Suspicious | Acellular | Not performed | Pleomorphic single-cell pattern |
| 8 | 88 | F | High-grade endometrial stromal sarcoma, uterus | Peritoneal | 0.7 | Positive | Clusters of large cells with prominent nucleoli and multilobulated nuclei | Positive: CD10 (patchy), ER (focal)
Negative: Caldesmon, S100, CK5, D2-40, WT1 |
Pleomorphic single-cell pattern |
| 9 | 39 | F | High-grade endometrial stromal sarcoma, uterus | Peritoneal | 5.7 | Positive | Acellular | Not performed | Pleomorphic single-cell pattern |
| 10 | 31 | M | Alveolar rhabdomyosarcoma, left foot soft tissue | peritoneal | 18.2 | Positive | A few small to intermediate-sized dyscohesive cells with high NC ratio and inconspicuous nucleoli | Positive: Desmin, myogenin
Negative: CD45, CD3, CD20, CD99 |
Small round blue cell pattern |
| 11 | 23 | F | No prior history of a neoplasm | peritoneal | N/A | Positive | Numerous small round blue cells with numerous mitoses and scattered tingible body macrophages | Positive: Desmin, myogenin, myo-D1, INI1, vimentin
Negative: CD45, CD3, CD20, CD79a, PAX5, TDT, CD10, CD43, TFE3, S100, CD99, AE1/AE3, CAM5.2, synaptophysin, CD56 |
Small round blue cell pattern |
| 12 | 27 | F | Embryonal rhabdomyosarcoma, vagina | peritoneal | 0.7 | Positive | Rare clusters of small to intermediate-sized cells with high NC ratio and rare prominent nucleoli | Positive: Desmin, myogenin, myo-D1, vimentin
Negative: CD45, CD3, CD20, AE1/AE3, |
Small round blue cell pattern |
| 13 | 66 | F | Alveolar rhabdomyosarcoma, ethmoid sinus | pleural | 13.1 | Suspicious | Acellular | Not performed | Small round blue cell pattern |
| 14 | 4 | F | No prior history of a neoplasm | pleural | N/A | Positive | Numerous dyscohesive small cells with high NC ratio, and granular chromatin | Positive: Vimentin, desmin, myogenin, Negative: CD45, CD3, CD20, CD99, S100, AE1/AE3, CAM5.2, synaptophysin | Small round blue cell pattern |
| 15 | 58 | M | Osteosarcoma, fibula | pleural | 16.8 | Suspicious | Acellular | Not performed | Small round blue cell pattern |
| 16 | 29 | M | Ewing’s sarcoma, shoulder soft tissue | pleural | 15.2 | Positive | Many small round blue cells and extensive necrotic debris | Positive: CD99, FLI-1, vimentin, synaptophysin
Negative: CD45, CK8/18, AE1/AE3 |
Small round blue cell pattern |
| 17 | 18 | F | Ewing’s sarcoma, chest wall | pleural | 0.7 | Positive | Aggregates of small round blue cells with crush artifact and necrosis | Positive: CD99, FLI-1
Negative: CD45, CD30, Desmin, AE1/AE3, synaptophysin, chromogranin |
Small round blue cell pattern |
| 18 | 17 | F | Ewing’s sarcoma, scapula | pleural | 15.0 | Positive | Many monomorphic small round blue cells | Positive: CD99, FLI-1
Negative: CD45, CD3, CD20, desmin, AE1/AE3 |
Small round blue cell pattern |
| 19 | 27 | M | Ewing’s sarcoma, submandibular gland | Pericardial | 87.1 | Positive | Clusters of large epithelioid cells with high NC ratio, coarse chromatin, and multiple prominent nucleoli | Positive: CD99, FLI-1 (weak)
Negative: CD45, CK5, Calretinin, AE1/AE3 |
Small round blue cell pattern |
Cases with Glandular Cell Pattern
Three cases of metastatic sarcoma in our study showed a glandular cell pattern in ThinPrep attributable to their epithelioid appearance and vacuolated cytoplasm. These include one case each of myxoid liposarcoma, osteosarcoma, and clear cell sarcoma. Their morphological features are illustrated in Figure 1. Myxoid liposarcoma presented as large atypical epithelioid cells with round to spindled nuclei, fine chromatin, prominent nucleoli, and abundant vacuolated cytoplasm (Figure 1A – case 1). In the cellblock, these cells were positive for desmin and S100 while negative for CK5, calretinin, and, AE1/AE3. Osteoblastic osteosarcoma showed clusters of epithelioid cells with coarse chromatin, irregular nuclear contours, prominent nucleoli, and a small amount of vacuolated cytoplasm (Figure 1B – case 2). Only scant neoplastic cells were found in the cellblock that was negative for AE1/AE3, MOC31, S100, D2-40, SMA, and CK5 excluding other entities in the differential diagnosis. Clear cell sarcoma showed cohesive clusters of large epithelioid cells with irregular nuclear contours, fine chromatin, prominent nucleoli, and abundantly clear to foamy cytoplasm (Figure 1C and D – case 3). In the cellblock, these cells were positive for HMB45, S-100, and vimentin while negative for AE1/AE3, MOC31, and CAM5.2, confirming the diagnosis. The clinical and pathologic findings of these 3 cases are summarized in Table 1 (cases 1-3).
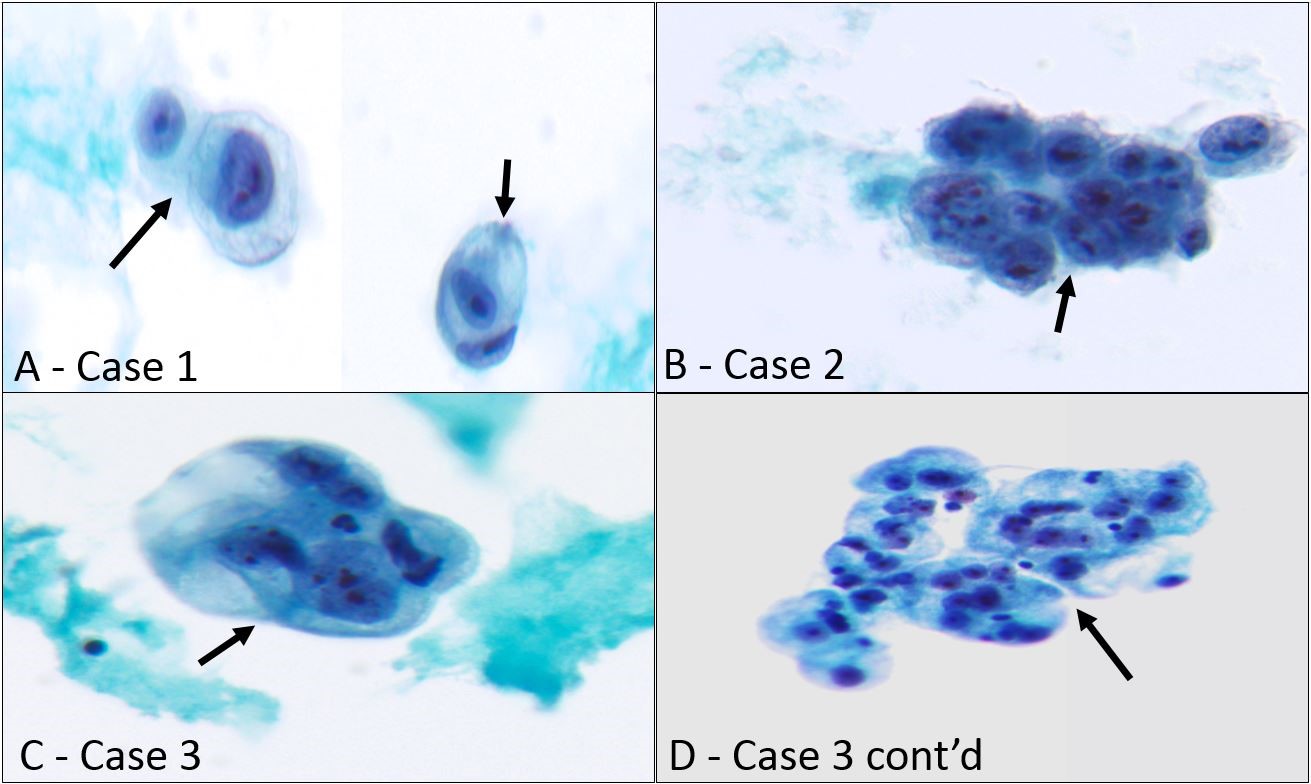
Figure 1: Sarcomas in body fluids showing glandular cell pattern.
- Myxoid liposarcoma involving pleural fluid (Case 1), large atypical epithelioid cells with round to spindled nuclei, fine chromatin, prominent nucleoli, and abundant vacuolated cytoplasm are seen (black arrows) (Papanicolaou stain, x400)
- Metastatic osteosarcoma in pleural fluid (Case 2), a cluster of large atypical cells with coarse chromatin, prominent nucleoli, and a small amount of vacuolated cytoplasm is shown (black arrow) (Papanicolaou stain, x400)
- Clear cell sarcoma involving pleural cavity (Case 3), a cohesive cluster of large epithelioid cells with irregular nuclear contours, fine chromatin, prominent nucleoli, and abundant clear cytoplasm is present (black arrow) (C-Papanicolaou stain, x400)
- Case 3 continued, with clear cell sarcoma in pleural fluid manifesting as atypical epithelioid cells with abundant foamy cytoplasm (black arrow) (D- Papanicolaou stain, x200)
Cases with Pleomorphic Single-cell Pattern
Six sarcoma cases in our study showed a pleomorphic single-cell pattern in body fluid ThinPreps characterized by their highly atypical and occasionally bizarre morphology. These include two cases each of high-grade leiomyosarcoma, osteosarcoma, and HG-ESS of the uterus. Their morphological features are shown in Figure 2. The two cases of leiomyosarcoma showed large highly atypical cells with hyperchromatic and often multilobulated nuclei with coarse chromatin (Figure 2 A and B – cases 4 and 5). A few spindle cells with centrally located ovoid and hyperchromatic nuclei and diffusely granular cytoplasm were also seen in one case (Figure 2A-blue arrows). Cellblock showed a few scattered bizarre cells as well as a few spindle cells that were positive for SMA and desmin in case 5 while case 4 was acellular.
The two cases of metastatic osteosarcoma in pleural fluid showed singly scattered highly atypical large cells with irregular and often eccentrically located nuclei, coarse chromatin, and prominent nucleoli (Figure 2 C and D – cases 6 and 7). One case showed abundant finely granular cytoplasm (Figure 2D-black arrows) as well as a few spindle cells with wrinkled hyperchromatic nuclei (Figure 2D-blue arrow). Cellblock was acellular in one case while the other showed occasional sarcomatoid cells. Both patients had biopsy-proven lung metastases. The two cases of uterine HG-ESS presented as highly atypical singly scattered and loosely aggregating large cells with irregular and often multilobulated nuclei, coarse chromatin, and prominent nucleoli (Figure 2 E and F – cases 8 and 9) in peritoneal cavity ThinPreps. Cellblock showed large neoplastic cells that were partially positive for CD10 and ER IHCs in one case while no neoplastic cells were found in the other. The clinical and pathologic findings of these 6 cases are summarized in Table 1 (cases 4-9).
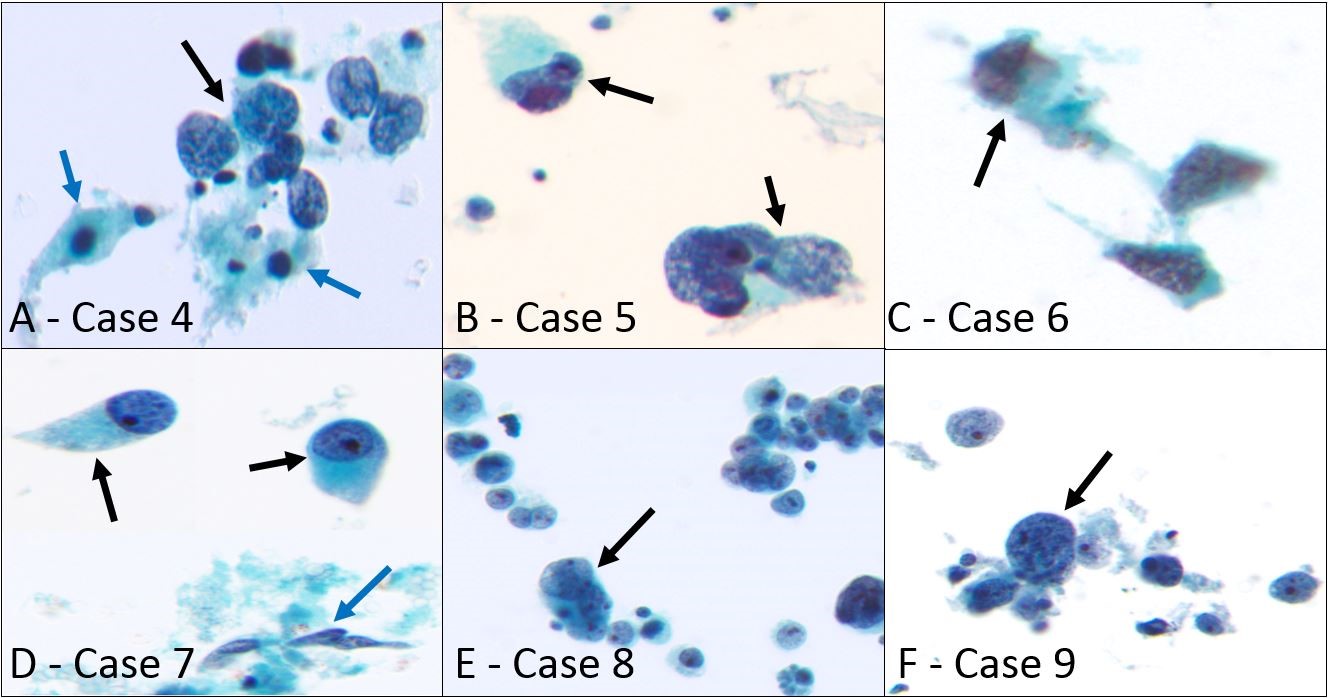
Figure 2: Sarcomas in body cavities showing pleomorphic single-cell pattern.
- High-grade leiomyosarcoma in ascitic fluid (case 4), large atypical cells with hyperchromatic and irregular nuclei, coarse chromatin, and stripped cytoplasm are shown (black arrow). In addition, a few spindle cells with centrally located ovoid and hyperchromatic nuclei and diffusely granular cytoplasm are also present (blue arrows) (Papanicolaou stain, x400)
- Another case of high-grade leiomyosarcoma in peritoneal washing specimen (case 5), two scattered large cells with bizarre multilobulated nuclei and coarse chromatin are present (black arrows) (Papanicolaou stain, x400)
- Metastatic osteosarcoma in the pleural cavity (case 6), a few scattered large cells with irregular nuclei, coarse chromatin, and high nuclear to cytoplasmic (NC) ratio are seen (black arrow) (Papanicolaou stain, x400)
- Another case of metastatic osteosarcoma in the pleural cavity (case 7), two singly scattered large atypical cells with eccentrically located round nuclei, coarse chromatin, prominent nucleoli, and abundant finely granular cytoplasm are present (black arrows). In addition, spindle cells with wrinkled hyperchromatic nuclei are also seen (blue arrow) (Papanicolaou stain, x400)
- High-grade endometrial stromal sarcoma in peritoneal washing specimen (case 8), numerous singly scattered as well as cohesive highly atypical large cells with multilobulated nuclei and prominent nucleoli are shown (black arrow) (Papanicolaou stain, x400)
- Another case of high-grade endometrial stromal sarcoma in ascitic fluid (case 9), scattered large atypical cells with high NC ratio, round to irregular nuclei, coarse chromatin, and prominent nucleoli are present (black arrow) (Papanicolaou stain, x400)
Cases with Small Round Blue Cell Pattern
Ten cases in our study showed a small round blue cell pattern characterized by singly scattered small to intermediate-sized cells with a high NC ratio. These include 5 cases of rhabdomyosarcoma (4 alveolar and 1 embryonal subtype), 4 ES, and 1 small cell variant of osteosarcoma. Figures 3 and 4 illustrate the morphologic findings of all 10 cases. ThinPrep in rhabdomyosarcoma cases showed singly scattered and rarely cohesive small cells with high NC ratio, round to slightly irregular nuclei, fine chromatin, and multiple pinpoint nucleoli (Figure 3 A-E – cases 10-14). One case showed scattered tingible body macrophages (Figure 3B-blue arrow), mitotic figures, and apoptotic debris. Cells with eccentric nuclei and dense cytoplasm suggestive of rhabdomyoblastic differentiation were only seen in one case (Figure 3D, red arrow). Two cases presented with a malignant effusion as their first presentation (cases 11 and 14). IHCs for desmin, myogenin, and myo-D1 on the cellblock aided in a definitive diagnosis in 4 cases while one case was acellular.
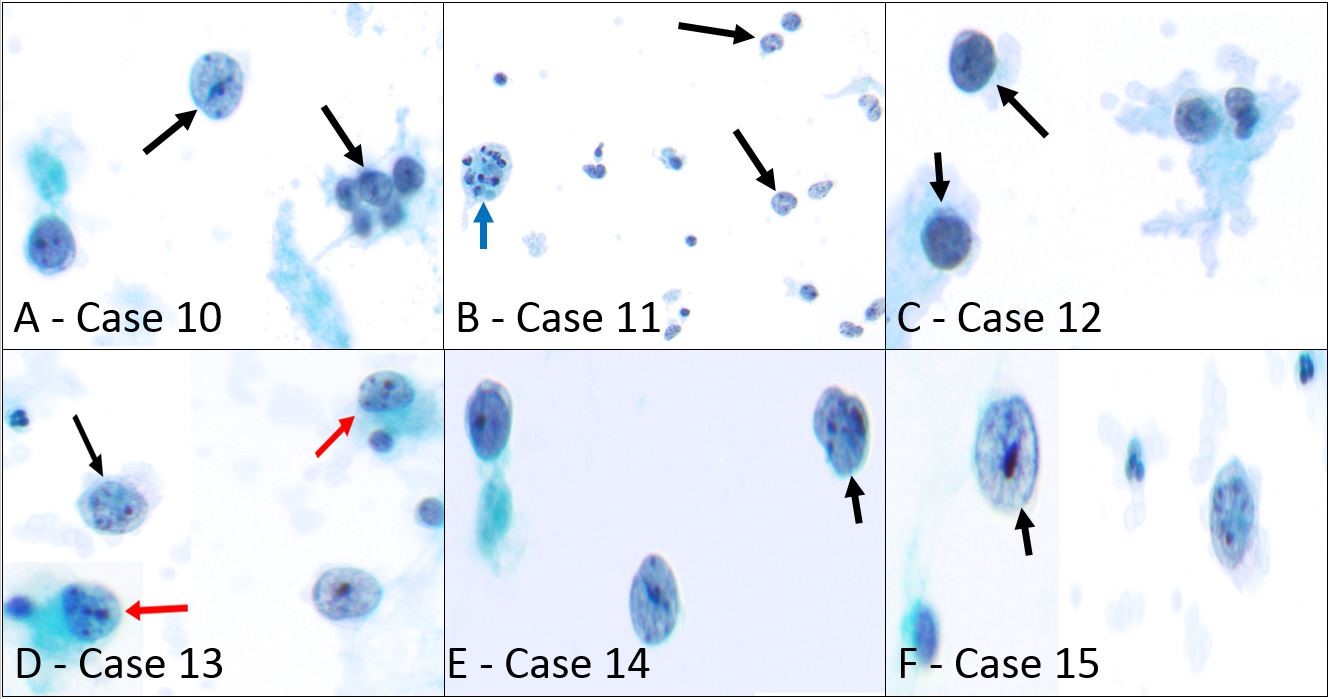
Figure 3: Sarcomas in body fluids showing small round blue cell pattern.
- Alveolar rhabdomyosarcoma in ascitic fluid (case 10), singly scattered and rarely cohesive small to intermediate-sized atypical cells with high NC ratio, round nuclei, fine chromatin, and multiple pinpoint nucleoli are shown (black arrow) (Papanicolaou stain, x400)
- Alveolar rhabdomyosarcoma presents with massive ascites (case 11), numerous dyscohesive intermediate-sized cells with high NC ratio, irregular nuclear contours, fine chromatin, and eccentric small nucleoli are present (black arrows). A tingible body macrophage can also be seen (blue arrow) (Papanicolaou stain, x200)
- Embryonal rhabdomyosarcoma in peritoneal fluid (case 12), scattered small to intermediate-sized cells with high NC ratio, round nuclei, clumped chromatin, and inconspicuous nucleoli can be seen (black arrows) (Papanicolaou stain, x400)
- Alveolar rhabdomyosarcoma involving pleural fluid (case 13), singly scattered small to intermediate-sized cells with high NC ratio, round to slightly irregular nuclear contours, fine chromatin, and prominent nucleoli are present (black arrow). In addition, two cells with eccentric nuclei and dense cytoplasm suggestive of rhabdomyoblastic differentiation are also seen (red arrows) (Papanicolaou stain, x400)
- Alveolar rhabdomyosarcoma involving pleural fluid (case 14), singly scattered small to intermediate-sized cells with high NC ratio, round to slightly irregular nuclear contours, fine chromatin, and pinpoint nucleoli are present (black arrow) (Papanicolaou stain, x400)
- Small cell variant of osteosarcoma in pleural fluid (case 15), singly scattered large cells with high NC ratio, irregular eccentric nuclei, fine chromatin, and prominent nucleoli are seen (black arrow) (Papanicolaou stain, x600)
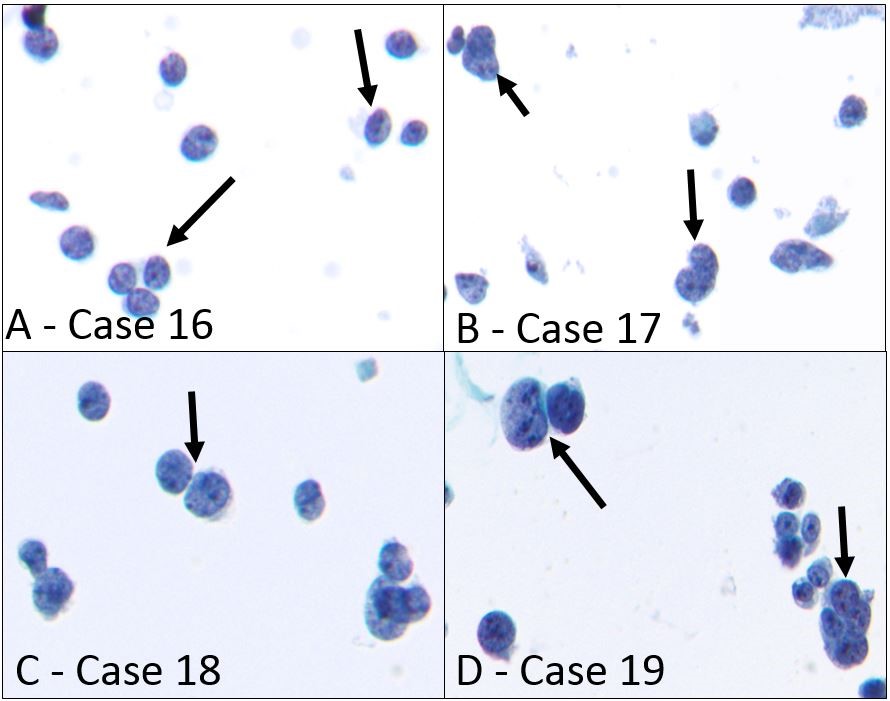
Figure 4: Ewing sarcoma in body fluids showing small round blue cell pattern.
- Ewing sarcoma involving pleural cavity (case 16), singly scattered small cells with high NC ratio, round to slightly irregular nuclear contours, granular chromatin, and inconspicuous nucleoli resembling lymphocytes can be seen (black arrows) (Papanicolaou stain, x400)
- Ewing sarcoma involving pleural cavity (case 17), small to intermediate-sized singly scattered cells with stripped cytoplasm, irregular nuclear contours, stippled chromatin, and rare small nucleoli are present (black arrows) (Papanicolaou stain, x400)
- Ewing sarcoma recurring as pleural effusion (case 18), loosely clustered as well as singly scattered intermediate-sized atypical cells with high NC ratio, round to irregular nuclear contours, finely granular chromatin, and inconspicuous nucleoli are shown (black arrow) (Papanicolaou stain, x400)
- Large cell variant of Ewing sarcoma in pericardial fluid (Case 19), singly scattered as well as clustering intermediate to large atypical cells with round nuclei, coarse chromatin, multiple nucleoli, and a small rim of coarse cytoplasm can be seen (black arrows) (Papanicolaou stain, x400)
Also included in the small round blue cell category were 4 cases of ES and 1 case of small cell osteosarcoma. The patient with osteosarcoma had a known history of the small cell variant and presented with bilateral pulmonary metastases as well as pleural effusion (Figure 3F – case 15). In ThinPrep it appeared as single scattered intermediate-sized cells with high NC ratio, fine chromatin, and prominent nucleoli (Figure 3F). Three of the four ES cases showed singly scattered small cells with high NC ratio, round to slightly irregular nuclear contours, granular chromatin, and inconspicuous nucleoli (Figure 4 A-C – cases 16-18). One case of large cell variant of ES presented with pericardial effusion and showed singly scattered and clustering intermediate to large atypical cells with round nuclei, coarse chromatin, multiple nucleoli, and a small rim of coarse cytoplasm (Figure 4F). IHCs for CD99 and FLI-1 aided in a definitive diagnosis in all 4 cases. The clinical and pathologic features of these 10 cases are summarized in Table 1 (cases 10-19).
Most existing literature describing the cytomorphologic features of sarcomas comes from FNA and TPs of targeted lesions hence, recognizing them in liquid-based cytologic preparations is challenging. This study describes the cytomorphologic features of sarcomas in liquid-based ThinPrep specimens from body cavity fluids in one of the largest series to date. Our study highlights the pronounced cytomorphologic heterogeneity shown by metastatic sarcomas in body cavity fluid ThinPrep specimens that often overlaps with other more common malignant entities. As illustrated in our study, sarcomas with glandular cell patterns show abundant vacuolated cytoplasm resembling well-differentiated adenocarcinomas. Sarcomas with pleomorphic single-cell patterns mimic other high-grade neoplasms such as melanoma, large cell lymphoma, and poorly differentiated carcinoma. Those with a small round blue cell morphology imitate neuroendocrine carcinomas and hematolymphoid tumors. Recognition of this vast morphologic spectrum shown by sarcomas in body fluid specimens combined with careful clinical correlation and judicious use of immunohistochemistry can help avoid these diagnostic pitfalls.
The most common sarcoma involving body fluid in our study was rhabdomyosarcoma, a malignant neoplasm of skeletal muscle origin. It is the most common mesenchymal neoplasm in children and young adults5. The two most common subtypes are embryonal and alveolar rhabdomyosarcoma. The embryonal type usually manifests in the first decade of life and typically involves the head and neck, genitourinary system, or trunk while the alveolar subtype is a disease of adolescents and young adults and is characterized by PAX3:FOXO1 or PAX7:FOXO1 translocations6. In FNA and TPs, both subtypes show moderate to high cellularity composed predominantly of isolated cells with a high NC ratio and significant nuclear pleomorphism. Some cells are described to have eccentric nuclei with inclusion like cytoplasmic condensations suggestive of rhabdomyoblastic differentiation. Other features of skeletal muscle differentiation such as cytoplasmic cross-striations and tadpole-shaped nuclei are only seen in rare instances7,8.
Our study includes 5 cases of rhabdomyosarcoma in body cavity fluids including 4 alveolar and 1 embryonal subtype. All 5 cases showed a small round blue cell pattern characterized by variably sized dyscohesive cells with a high NC ratio. All 4 cases of alveolar rhabdomyosarcoma displayed small to intermediate-sized cells with fine chromatin (Figure 3, A, B, D, E). 3 cases showed very small peripherally located nucleoli while one case showed multiple prominent nucleoli. Cells with eccentric nuclei and dense cytoplasm suggestive of rhabdomyoblastic differentiation were only seen in 1 case (Figure 3D-red arrow). Additionally, one case showed a background rich in apoptotic debris, mitotic figures, and tingible body macrophages (Figure 3B). One case of embryonal rhabdomyosarcoma showed very blandly appearing singly scattered cells with a high NC ratio, clumped chromatin, and inconspicuous nucleoli, resembling lymphocytes (Figure 3C). The cellblock was adequate in 4/5 cases and immunoreactivity for desmin, myogenin, and myo-D1 confirmed the diagnosis in all 4 cases. In cases where cellblock is not available or IHC is inconclusive, fluorescence in situ hybridization (FISH) analysis for FOXO1 can be performed on the body fluid for alveolar rhabdomyosarcoma. Interestingly, 2 cases of alveolar rhabdomyosarcoma (1 pleural and 1 peritoneal) had no prior history, and both patients presented with large effusions as their first manifestation. Other reports of atypical manifestations of alveolar rhabdomyosarcoma as body cavity effusions and leukemic phase have been described in the literature9-12.
The second most common sarcomas involving body cavity fluids in our study were metastatic osteosarcoma and ES (4 cases each). Osteosarcomas cause malignant effusions in exceedingly rare instances. In FNA and TP specimens, they have been described to show a wide spectrum of morphologic findings ranging from anaplastic highly pleomorphic single cells to cohesive clusters and primitive neuroectodermal tumor (PNET) like patterns in the small cell variant13,14.
Romanowsky stains such as Giemsa and Diff-Quik on cytologic preparations from tissue biopsies are extremely helpful in finding osteoid matrix material. Diagnosing osteosarcomas in body cavity fluids, however, is extremely challenging due to their highly variable morphology. Furthermore, the osteoid matrix material may be absent in ThinPrep specimens and when present may be difficult to recognize on Pap stain. Two out of four cases in our study showed a pleomorphic single-cell pattern with coarse chromatin (Figure 2C and D) resembling a poorly differentiated carcinoma. One case showed a glandular cell pattern (Figure 1B) resembling metastatic adenocarcinoma. The fourth case was a small cell variant of osteosarcoma and showed a small round blue cell pattern mimicking a PNET or a lymphoma (Figure 3F). The cellblock failed to reveal any distinguishable osteoid in all 4 cases. IHC has limited utility in the diagnosis of osteosarcomas as there are no specific markers and many osteosarcomas can show heterogenous immunophenotypes which overlap with other sarcomas, as well as carcinomas, melanomas, and mesotheliomas15. Due to these limitations, knowledge of the past medical history and clinical findings is critical in reaching the correct diagnosis.
Our study includes 4 cases of ES which is the second most common malignant bone tumor in children and young adults that commonly metastasizes to the lungs. However, malignant effusions due to ES are rare and occur in less than 10% of the patients16. In cytologic specimens, classic ES typically displays a small round blue cell pattern characterized by dyscohesive and loosely cohesive cells that are larger than a small lymphocyte with a high NC ratio and show finely granular chromatin and inconspicuous nucleoli. Some cells display nuclear molding and rarely pseudorosettes can be seen17. The rare large cell/atypical variant of ES comprises only 5% of all cases and as the name implies shows larger and more pleomorphic cells18. CD99 and FLI1 IHCs combined with molecular testing for EWSR1 rearrangements can distinguish ES from other small round blue cell tumors in nearly all cases. Three out of four cases in our study involved the pleural cavity. All 3 displayed the classic small round blue cell morphology showing singly scattered and loosely cohesive small to intermediate-sized single cells with high NC ratio, round to irregular nuclei, finely granular/stippled chromatin, and inconspicuous nucleoli (Figure 4, A-C). Two cases showed possible nuclear molding (Figure 4 C and D, black arrows). This study also includes one case of atypical/large cell ES involving pericardial fluid. This case showed large atypical scattered and cohesive cells with coarse chromatin, multiple nucleoli, and a small rim of coarse cytoplasm (Figure 4D). IHCs for CD99 and FLI-1 on the cellblock aided in a definitive diagnosis in all 4 cases.
This study also includes 4 cases of uterine sarcomas involving the peritoneal cavity. These include two cases each of high-grade leiomyosarcoma and HG-ESS. Peritoneal cavity involvement is seen in uterine sarcomas in less than 10% of cases with the most common subtype being leiomyosarcoma followed by endometrial stromal sarcoma and adenosarcoma19,20. All 4 cases of uterine sarcomas in our study showed a pleomorphic single-cell pattern resembling poorly differentiated carcinoma. One case of leiomyosarcoma showed naked nuclei and occasional spindle cells with centrally located oval, pyknotic, and hyperchromatic nuclei and diffusely granular cytoplasm resembling a squamous cell carcinoma (Figure 2A). Cohesive clusters and fascicles of spindle cells with rigid edges described in FNA and TP specimens were not seen in the ThinPreps21. Both cases of HG-ESS showed singly scattered and loosely clustered highly atypical cells with coarse chromatin and multiple prominent nucleoli. One case showed occasional cells with large multilobulated nuclei and dense cytoplasm (Figure 2E) while the second case showed cells with large monolobate nuclei with stripped cytoplasm (Figure 2F). Lastly, there was 1 case each of myxoid liposarcoma and clear cell sarcoma involving pleural fluid. Both showed a glandular cell pattern. The ThinPrep in myxoid liposarcoma showed lipoblasts with abundant vacuolated cytoplasm (Figure 1A) mimicking a well-differentiated adenocarcinoma. The granular myxoid matrix and delicate thin-walled capillaries seen in cytologic preparations from tissue biopsy specimens were not seen in the ThinPrep22. Metastatic clear cell sarcoma manifested as singly scattered and a few tightly cohesive clusters of epithelioid cells with prominent nucleoli and abundantly clear to foamy cytoplasm also resembling a well-differentiated adenocarcinoma (Figure 1C and D). Due to the distinctive immunoprofile (HMB45 and S-100 positivity) a diagnosis of clear cell sarcoma was easily made on the cellblock.
Given this extensive cytomorphologic heterogeneity and the rarity of sarcomas in body cavity fluids, careful correlation with clinical history, presentation, imaging features, concurrent histology, immunophenotype, and cytogenetic/molecular findings is crucial for accurate diagnosis
In summary, sarcomas involving body cavity fluids display a broad spectrum of morphologic findings that can mimic other neoplasms and pose a significant diagnostic challenge. Rhabdomyosarcomas typically display a dyscohesive small round blue cell pattern and can rarely show rhabdomyoblasts. Metastatic osteosarcomas display highly variable morphology ranging from highly pleomorphic large cells to small round blue cells in the small cell variant. ES predominantly manifests as small round blue cells but can show large atypical cells with prominent nucleoli in the large cell variant. High-grade leiomyosarcomas and HG-ESS in ThinPrep body cavity fluids show highly pleomorphic large cells while clear cell sarcoma and myxoid liposarcoma show cohesive clusters of epithelioid cells with abundant clear cytoplasm resembling well-differentiated adenocarcinomas. Given the vast cytomorphologic heterogeneity and the rarity of sarcomas involving body cavity fluids, careful correlation with clinical history, presentation, imaging features, concurrent histology, immunophenotype, and cytogenetic/molecular alterations is crucial for accurate diagnosis.
We would like to acknowledge all co-authors for their contributions to this manuscript.
The authors have no competing interests to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
HT contributed to the conception, design, data collection, and drafting of the manuscript. SSM and NV assisted in data collection and study design. AA also contributed to data collection while WM and SR did the critical revision of the manuscript. Furthermore, PG and LC contributed to the study conception and design, data collection, and critical revision of the manuscript.
- Arnold DT, De Fonseka D, Perry S, Morley A, Harvey JE, Medford A, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J. 2018;52(5):1-9. doi: 10.1183/13993003.01254-2018
- Sears D, Hajdu SI. The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31(2):85-97.
- Ehya H. Effusion cytology. Clin Lab Med. 1991;11(2):443-467. doi: 10.1016/S0272-2712(18)30563-8
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30. doi: 10.3322/caac.21166
- Rudzinski ER, Anderson JR, Chi YY, Gastier‐Foster JM, Astbury C, Barr FG, et al. Histology, fusion status, and outcome in metastatic rhabdomyosarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2017;64(12):1-7. doi: 10.1002/pbc.26645
- Skapek SX, Ferrari A, Gupta AA, Lupo PJ, Butler E, Shipley J, et al. Rhabdomyosarcoma. Nat Rev Dis Primers. 2019;5(1):1-9. doi: 10.1038/s41572-018-0051-2
- Klijanienko J, Caillaud JM, Orbach D, Brisse H, Lagacé R, Vielh P, et al. Cyto‐histological correlations in primary, recurrent and metastatic rhabdomyosarcoma: the institut Curie’s experience. Diagn Cytopathol. 2007;35(8):482-487. doi: 10.1002/dc.20662
- Pohar-Marinsek Z, Bracko M. Rhabdomyosarcoma. Cytomorphology, subtyping and differential diagnostic dilemmas. Acta Cytol. 2000;44(4):524-532. doi: 10.1159/000328524
- Kida Y, Katakami N, Tomii K, Ishihara K, Takahashi Y, Imai Y. Case of rapidly progressing primary pulmonary rhabdomyosarcoma with bloody pleural effusion. Nihon Kokyuki Gakkai Zasshi. 2009;47(5):404-409.
- Yozgat C, Yesilbas O, Yozgat Y, Akdemir OC, Yurtsever I, Tekin N, et al. Persistent pleural effusion in an infant with an unusual diagnosis: congenital alveolar rhabdomyosarcoma. Contemp Oncol (Pozn). 2020;24(2):132-138. doi: 10.5114/wo.2020.97639
- Klouda T, Steele J, Weichert‐Leahey N, Mack J, Subramaniam M. Alveolar rhabdomyosarcoma presenting as a pleural effusion: An atypical presentation of a malignancy. Pediatr Pulmonol. 2020;55(12):3231-3233. doi: 10.1002/ppul.25110
- Putti MC, Montaldi A, d’Emilio A, Sainati L, Milanesi C, Stella M, et al. Unusual leukemic presentation of rhabdomyosarcoma: Report of two cases with immunological, ultrastructural and cytogenetical studies. Haematologica. 1991;76(5):368-374.
- Sathiyamoorthy S, Ali SZ. Osteoblastic osteosarcoma: cytomorphologic characteristics and differential diagnosis on fine-needle aspiration. Acta Cytol. 2012;56(5):481-486. doi: 10.1159/000339196
- Domanski HA, Åkerman M. Fine‐needle aspiration of primary osteosarcoma: A cytological‐histological study. Diagn Cytopathol. 2005;32(5):269-275. doi: 10.1002/dc.20240
- AbdullGaffar B. Osteosarcoma pleural effusion: A diagnostic challenge with some cytologic hints. Diagn Cytopathol. 2021;49(1):E40-44. doi: 10.1002/dc.24569
- Frankel D, Kaspi E, Bouvier C, Roll P. Pleural effusion in a patient with Ewing sarcoma. Cytopathology. 2022;33(1):138-140. doi: 10.1111/cyt.12989
- Guiter GE, Gamboni MM, Zakowski MF. The cytology of extraskeletal Ewing sarcoma. Cancer Cytopathol. 1999;87(3):141-148. doi: 10.1002/(SICI)1097-0142(19990625)87:3<141:AID-CNCR7>3.0.CO;2-C
- Renshaw AA, Perez-Atayde AR, Fletcher JA, Granter SR. Cytology of typical and atypical Ewing’s sarcoma/PNET. Am J Clin Pathol. 1996;106(5):620-624. doi: 10.1093/ajcp/106.5.620
- Matsuo K, Matsuzaki S, Nusbaum DJ, Ki S, Chang EJ, Klar M, et al. Significance of malignant peritoneal cytology on survival of women with uterine sarcoma. Ann Surg Oncol. 2021;28(3):1740-1748. doi: 10.1245/s10434-020-09202-1
- Wang X, Khoo US, Xue WC, Cheung AN. Cervical and peritoneal fluid cytology of uterine sarcomas. Acta Cytol. 2002;46(3):465-469. doi: 10.1159/000326862
- Klijanienko J, Caillaud JM, Lagacé R, Vielh P. Fine‐needle aspiration of leiomyosarcoma: a correlative cytohistopathological study of 96 tumors in 68 patients. Diagn Cytopathol. 2003;28(3):119-125. doi: 10.1002/dc.10249
- Layfield LJ, Dodd L, Klijanienko J. Myxoid neoplasms of bone and soft tissue: a pattern-based approach. J Am Soc Cytopathol. 2021;10(3):278-292. doi: 10.1016/j.jasc.2020.09.009
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
