By Ramsha Akhtar1, Muhammad Rohail Akhtar2, Rabia Arshad3, Sabeen Masood4
AFFLIATIONS:
- Department of Dental Material, Birmingham University, United Kingdom (UK) and Department of Dental Material Sciences, Bolan Medical College, Quetta, Pakistan.
- Islamic International Dental College, Riphah International University, Islamabad, Pakistan.
- Department of Pharmacology, Altamash Institute of Dental Medicine, Karachi, Pakistan.
- Department of Operative Dentistry, Altamash Institute of Dental Medicine, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-3/002
ORCID iD: 0000-0001-5805-7163
How to cite: Akhtar R, Akhtar MR, Arshad R, Masood S. Evaluation of Antibiotic Susceptibility Tests: Agar Diffusion and Broth Dilution Assay Against Enterococcus faecalis. Pak J Med Dent. 2022;11(3): 3-10. doi: 10.36283/PJMD11-3/002
Background: Antibiotic susceptibility testing (AST), a technique to measure antibiotic susceptibility to different infections, is used for drug invention, estimation of therapeutic outcomes, and evaluation of their ability to withhold bacterial growth. This study aimed to compare the antibiotic susceptibilities of various important antibiotics using agar diffusion and broth dilution assays against the growth of Enterococcus faecalis (E. faecalis).
Methods: This experiment was carried out in the Microbiology Laboratory at the Birmingham Dental Hospital, Birmingham. In the Agar Diffusion Assay, different solutions of concentrations (50mg/ml), punch into nutrient agar dishes in two groups, n=15(peer A1) and n=15 (peer A2), inoculated with strains E. faecalis. Inhibitory zones were measured under European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. While in the Broth assay, bacteria were inoculated in 100 µL in the presence of multiple concentrations of antimicrobial solutions and bacterial growth was assessed using Optical Densities (OD) measurement.
Results: Agar diffusion assay showed the susceptibility of E. faecalis against Ampicillin, Gentamycin, and Erythromycin (OD< 0.1), whereas, it was found resistant with no zone of inhibition by Metronidazole (OD > 0.1). Similarly, broth dilution assay resulted in marked E. faecalis susceptibility to Ampicillin and Gentamycin at a minimum inhibitory concentration (5 mg/dl and 0.5mg/dl), but was not responsive to Metronidazole. When compared statistically with peer A2 non-significant values were obtained for Gentamycin, Ampicillin, and Erythromycin (p-value=0.5, 0.28, 0.23 respectively).
Conclusion: Antibiotics susceptibility measured by Broth Dilution Assay showed more authentic results in terms of minimum inhibitory concentration and optic density compared to Agar Diffusion Assay.
Keywords: Bacterial Sensitivity Tests; Microbiological Techniques; Serum Bactericidal Test; Minimum Inhibitory Concentration; Enterococcus faecalis; Zone Diameter Breakpoints.
In the previous few years, a lot of work has been done for proper diagnosis and management of infections by numerous newer techniques, such as the agar diffusion method, well diffusion assay, broth dilution assay, and Epsilometer tests1. Antibiotic susceptibility testing (AST) defines adequate antibiotic dosage to effectively manage bacterial infections. Antimicrobial susceptibility testing can be used for drug invention, estimation of therapeutic outcomes, and evaluation of their ability to withhold bacterial growth, further clarified by the minimum inhibitory concentration (MIC) 2.
The Agar diffusion test is the core and essential method for determining antimicrobial activity3. This assay is used for the estimation of MIC by measuring the diameter inhibitory zone in the surroundings of the applied antibiotic disc with grown microbe culture4,5. For the Broth micro-dilution assay, the microbial solution is injected with sequential dilutions of antibiotic agents to determine the minimum inhibitory concentration (MIC) 6.
Moreover, Enterococcus faecalis is strongly positive for staining with Gram stain and is occurring naturally in the gastrointestinal tract7. But if it grows abundantly it spread to other body areas and can lead to life-threatening infections. People with low immunity or with a prolonged stay at the hospital are at the highest risk of having a particular infection. Centers for Disease Control and Prevention (CDC) has confirmed that E. faecalis is responsible for about 80% of gut-related problems and frequently has been detected in more than 30% of oral and root canal cases8. Treated root canals are about nine times more than cases of primary infections infected with E. faecalis 9,10. As it is a commonly occurring infectious agent that requires early diagnosis and prompt treatment, the current study was proposed to consider and compare agar diffusion and broth dilution assays to estimate the antibiotic inhibitory ability against the growth of E. faecalis in vitro along with an estimation of minimum inhibitory concentrations. The objective of the study was to evaluate the susceptibility of E. faecalis against four antibiotics, gentamycin, erythromycin, ampicillin, and metronidazole by comparing agar diffusion and broth dilution assays methods.
The experiment was carried out in the Microbiology Lab of the Birmingham Dental Hospital in two groups n=30 [peer A1(15), peer A2(15)]. First, for the Agar diffusion assay, solutions of Gentamycin, Ampicillin, erythromycin, and Metronidazole of equal strength (50mg/mL) are pressed into nutrient agar dishes inoculated with a strain of E. faecalis bacteria. Following the incubation for approximately 20 hours, antibiotic dispersion from a disc into the agar causes cessation of microbial growth in the surroundings of each tablet of antibiotic, also known as inhibitory clear zones as no bacterial growth. This is centered on the impression that antibiotics spread easily in the solid nutrient medium. After this process, the clear zones are measured around each of the wells. Interpretation of the zones done following EUCAST Guidelines5.
In Broth dilution assay, a 96-microtiter well plate is used to inoculate the same strains of bacteria with the 100uL of BHI Broth growth medium in the presence of variable concentrations of Gentamycin, Ampicillin, and Metronidazole. Further growth of the bacteria was assessed after incubation for 16-20 hours.
For Optic Density Measurement, in the dilution test, E. faecalis was confirmed with generating apparent visual growth in solution, with several strengths of the microbial agents (5-0.00005 mg/mL). Minimum inhibitory concentration (MIC) was computed by an automated Universal Microplate-plate reader (BioTek Instruments EL x 800, Gen5 Software) at OD600nm and the required breakpoint concentration was determined as indicated in ISO Standard. The results of A1 and peer A2 were compared and statistical analysis was done using an independent students t-test and a p-value <0.05 was considered statistically significant.
The Agar diffusion assay in assessing the susceptibility of four antibiotic solutions demonstrated that E. faecalis is susceptible to gentamycin, Ampicillin (B-Lactam antibiotic), and erythromycin in vitro with the mean zone of inhibition measuring 22.8, 34.7, 32.8 mm respectively. All the zones of inhibition of the above-stated drugs were above 22mm thus giving susceptible results. No drug showed a zone between 18-22mm whereas metronidazole showed a zone of inhibition less than18 mm and thus had a resistant pattern as per new EUCAT guidelines for agar diffusion assay breakpoint (Table 1A).
Table 1A: Three different antibiotic susceptibilities against E. faecalis were measured by the Agar diffusion assay method.
| Antibiotics susceptibility | Enterococcus faecalis (n=15) | Phosphate-buffered saline (PBS) controls | |||
| Anti-
microbial agent |
G | A | E | M | |
| The volume of antibiotics (μL) | 50 | 50 | 50 | 50 | 50 |
| Concentration
(mg/ml) |
50mg/ml | 50mg/ml | 50mg/ml | 25mg/ml | – |
| Static Temperature
(C) for incubation |
37 | 37 | 37 | 37 | 37 |
| Duration for incubation
(h) |
20 | 20 | 20 | 20 | 20 |
| Mean inhibition zone
diameter (mm) |
22.8 | 34.7 | 32.8 | 0 | 0 |
| EUCAST Agar diffusion assay breakpoints | |||||
| Susceptible therapeutic dose
(S>22mm) |
E. faecalis susceptible |
E. faecalis susceptible |
E. faecalis susceptible |
– |
– |
| Susceptible increased exposure Intermediate
(I= 18-21mm) |
– |
– |
– |
– |
– |
| Resistant
(R<18mm) |
– | – | – | E. faecalis resistant | – |
EUCAST GUIDELINE: Antimicrobial Susceptibility Testing guidelines by European Committee, Breakpoint tables for interpretation of MICs and zone diameters; S=Susceptible (S>22mm), I= Intermediate (I= 18-21mm), R=Resistant (R<18mm), OMG: Oral microbiology group, G-Gentamycin, A-Ampicillin, E-Erythromycin, M-Metronidazole, PBS: Phosphate buffer saline.
On the other hand, in broth dilution assay bacteria were inoculated with varying concentrations of three antimicrobial agents (Gentamycin, Ampicillin, and Metronidazole). All samples of E. faecalis showed susceptibility to Ampicillin, and Gentamycin as optic density was greater than 0.1 but was not responsive to metronidazole as presented optic density was less than 0.01. The mean minimum inhibitory concentration was evaluated to be 5 mg/dl and 0.5mg/dl for ampicillin and gentamicin respectively (Table 1B).
Table 1B: Minimum inhibitory concentration (MIC) and Optical Density Analysis.
| Clinical bacterial isolates | Enterococcus faecalis (n=15) | Phosphate-buffered saline (PBS) controls | ||
| Medium | Brain and heart infusion broth/
BHI |
|||
| Antibiotic (mg/mL) | A | G | M | |
| Minimum inhibitory concentration
(mg/mL) mean |
5 mg/mL | 0.5 mg/mL | nil | – |
| Optical density
(OD 600 nm) |
0.08 | 0.11 | >0.1 | >0.1 |
| EUCAST MIC breakpoints description
Optical density |
<0.1 | <0.1 | > 0.1 | – |
| Bacterial growth | nil | nil | Present | |
| E. faecalis susceptibility
to a standard therapeutic dose of antimicrobial agent |
Susceptible 5mg/ml | Susceptible, 0.5mg/ml | Resistant | Controls contaminated |
G-Gentamycin, A-Ampicillin, M-Metronidazole.
Similar results were observed by peer A2 (Table 2). The results of peer A1 and peer A2 were compared and statistical analysis was done for A1 vs A2, and no significant values were obtained in terms of E. faecalis susceptibility to Gentamycin, ampicillin, erythromycin (p-value: 0.5, 0.28, 0.23 respectively) whereas metronidazole did not show any inhibition whether agar diffusion or broth dilution assay was utilized. Identical results of both peers signified that the said bacteria are sensitive to the three antibiotic groups but resistant to metronidazole because no zone of inhibition nor any MIC was found during the experiment (Figure 1).
Table 2: Comparative analysis between peer A1/A2 using E. faecalis (N=30) microbial isolates.
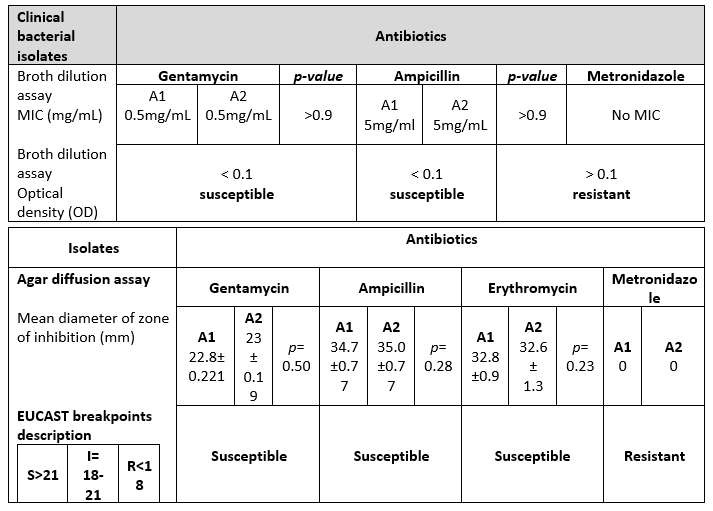
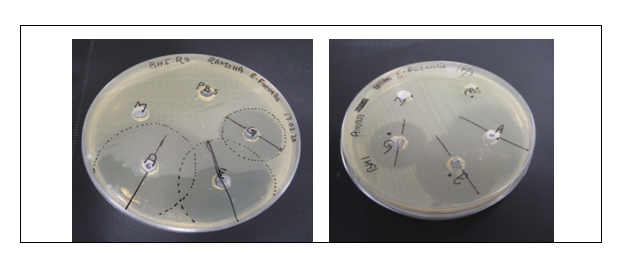
Figure 1: Agar well diffusion susceptibility test marked zones of inhibition.
The current approach for testing antibiotic susceptibility has been aimed at two basic doctrines. First, according to the inhibition zones formed by antibiotics active against Enterococcus faecalis on an agar plate in disc diffusion assay11. Further, disk diffusion is a precise and accurate method for conducting sensitivity in which the EUCAST method is considered widely acceptable12. The second method is broth dilution assay, in which the AST is based is the minimum inhibitory concentration of antibiotics, and their optical densities are used in the experiment. MIC method is used in resistance monitoring and comparative testing of antimicrobial agents13. It is determined from the results that the isolate of Enterococcus faecalis is susceptible to the therapeutic dose of Ampicillin as there were significantly clear halo zones showing growth inhibition agar diffusion and the same was true for dilution series. Widely used ampicillin has also shown proven results with good susceptibility recently in another study for E. faecalis14.
Similarly, aminoglycosides also prevented the growth of the bacterial colonies in both assays and thus were highly sensitive. Few pieces of research indicated contrary to the results that Enterococci have shown resistance against aminoglycosides (gentamycin) and can be used to inspect for aminoglycoside resistance strains by (HLAR) 15. This study’s results specified that E. faecalis is susceptible to erythromycin, and these were inconsistent with the study documented by Kaushik et al., they found this drug potent in comparison with ciprofloxacin and minocycline for treating root canal infections caused by Enterococci16.
According to another study erythromycin also works best in synergism with aminoglycosides in the eradication of these microbes17. Whereas Erythromycin also impedes E. faecalis at standard therapeutic doses contrary to the action of metronidazole on the bacteria18. However, the E. faecalis isolates susceptibility to Metronidazole was not predicted by any of the tests. The comparative analysis of both AST methods using the same antibiotics against E. faecalis was done when a similar study was carried out by peer A2. The probable reason for the failure of metronidazole could be that the specific enzyme called Nitroreductases synthesized by these Enterococcus strains inhibits the activation of metronidazole, therefore, reducing the antimicrobial activity of the drug19.
Antibiotics susceptibility measurements by Broth Dilution Assay proved more accurate as focused on determining the values of MIC. Enterococcus isolates revealed <0.1 optical density measurement, therefore susceptible to at least inhibitory concentration of Gentamycin and Ampicillin whereas no MIC value was obtained against Metronidazole, hence allowing bacterial growth. The growth of E. faecalis hampers by Aminoglycoside (Gentamycin) at standard therapeutic doses according to the test results contrary to the outcomes documented by Bhat et al., in an Indian study20. Similarly, ampicillin and erythromycin actively inhibited bacterial growth in broth dilution series same as predicted in the study results by Conceição et al 21.
Furthermore, the antibiotics susceptibility testing using both assays with regards to their pros and cons marks that, the agar diffusion assay is utilized broadly to assess the antimicrobial efficacy of plants and microbial extracts and is also used as an alternative to the agar and broth tube dilution methods22. The Agar diffusion test is qualitative, easy to perform, and simple, and bacterial growth can be determined below the nano-fibrous scaffold (zone of inhibition). Whereas, the broth dilution technique indicates the amount of drug necessary to inhibit the bacteriostatic and bactericidal activity of tested microbes. It is used as a quantitative test for the evaluation of the antimicrobial efficacy of nano-fibrous fibrous scaffolds. This test is widely used to determine the minimum inhibitory concentrations (MICs) of antimicrobial agents.
Elaborating further, some of the negative aspects of the assay, for example, the usage and application of agar diffusion are hampered when multiple factors affect the inhibition zones diameters which alter the results of the study, including inoculum entity, incubation time, temperature, depth of agar, well space, etc. Similarly, the broth dilution test is time-consuming and tedious. In this test, an aliquot of bacterial inoculums is taken in a growth media and is completely absorbed into test samples which are technique sensitive23. Furthermore, there is a high chance of overlapping of the inhibition zones in the agar method which makes the measurement difficult and challenging hence producing the error in the readings. Whereas, the broth dilution is technically sensitive and therefore not validated for clinical trials. In this study, several errors during the process, such as doubtful sterilization of PBS and pipette, might have contaminated the control groups as the Optical density (OD) results differ. Therefore, controls were not utilized for further assay. In contrast to this, PBS does not generate a free zone in well diffusion assay was uncontaminated. Further, the solution of Erythromycin was precipitated resulting in only three antibiotics being used in the broth dilution assay.
Numerous alternative strategies have been used to overcome the problem of antibiotic resistance, produce better treatment outcomes, and successful elimination and prevention of bacterial infections in the host body. The latest modalities include bacteriophage therapy in which bacteriophage viruses are used to treat bacterial infection and render potential solutions in fighting against AMR24. Secondly, microbiologists have scrambled another weapon to combat antibiotic resistance by using another bacterium called predatory bacteria mostly Bdellovibrio bacteriovorus which are free-living and harmless to humans25. On the other hand, bacteriocins are strong candidates to be used as future therapeutic agents, showing antimicrobial efficacy in vitro models by exerting a positive immune response in the host body26. Furthermore, the introduction of probiotics, prebiotics, or synbiotics into the human diet is good for intestinal flora, leading to a reduction in bacterial-induced infection and acting as immune booster substances. Therefore, antibiotics susceptibility measured by Broth Dilution Assay renders more authentic results in terms of minimum inhibitory concentration and optic density. Agar diffusion assay in which zones overlap and excessive diffusion of antibiotics into the medium results in discrepancies (Figure 2).
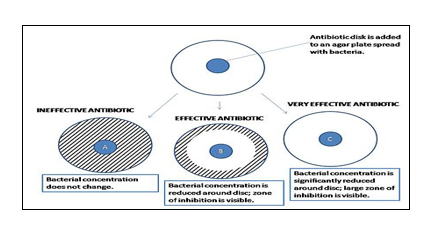
Figure 2: Measuring zones of inhibition27, 28.
Agar diffusion test confirmed Gentamycin, Ampicillin, and Erythromycin susceptibility for E. faecalis but resistance was observed against Metronidazole. Moreover, Broth dilution assay test comparatively works better and further confirmed susceptibility to similar antibiotics with effective inhibition of E. faecalis at Minimum Inhibitory level marked 5mg/mL for Ampicillin and 0.5mg/mL for Gentamycin but showed resistance against Metronidazole.
The authors would like to thank the Microbiology Laboratory at the Birmingham Dental Hospital, Birmingham for the facilitation and smooth conduction of the research.
The authors declared no conflict of interest.
The study was approved by the ethics review board of the Birmingham Dental Hospital, Birmingham.
RA conceptualized and designed the study, collected data and transcribed the manuscript. MRA analyzed and interpreted data. RA also drafted the manuscript and proofread the final paper, and SM reviewed the literature, and proofread the complete manuscript.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-79. doi: 10.1016/j.jpha.2015.11.005
- Wikler MA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. CLSI (NCCLS). 2006;26:M7-A7.
- Bauer AW. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966;45:149-158.
- Marston A. Thin-layer chromatography with biological detection in phytochemistry. J Chromatogr A. 2011;1218(19):2676-2683. doi: 10.1016/j.chroma.2010.12.068
- Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Odenholt I, et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect. 2006;12(6):501-503. doi: 10.1111/j.1469-0691.2006.01454.x
- Ge B, Wang F, Sjölund-Karlsson M, McDermott PF. Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. J Microbiol Methods. 2013;95(1):57-67. doi: 10.1016/j.mimet.2013.06.021
- de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap Adv Gastroenterol. 2018;11:1-11. doi: 10.1177/1756284818783606
- Ryan KJ, Ray CG. Medical microbiology. McGraw Hill. 2004; 379 p. Available from: https://booksdo.com/wp-content/uploads/XPreview/Pharmacology/3/sherris-medical-microbiology-7th-edition-by-kenneth-j-ryan.pdf
- Molander A, Reit C, Dahlen G, Kvist T. Microbiological status of root‐filled teeth with apical periodontitis. Int Endod J. 1998;31(1):1-7. doi: 10.1046/j.1365-2591.1998.t01-1-00111.x
- Rôças IN, Siqueira Jr JF, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30(5):315-320. doi: 10.1097/00004770-200405000-00004
- Jones RN. Recent trends in the College of American Pathologists proficiency results for antimicrobial susceptibility testing: preparing for CLIA’88. Clin Microbiol Newsl. 1992;14(5):33-37. doi: 10.1016/0196-4399(92)90033-6
- Ericsson HM, Sherris JC. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand. 1971(Suppl. 217): 90 p.
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(1):5-16. doi: 10.1093/jac/48.suppl_1.5
- Conceição N, Rodrigues WF, de Oliveira KL, da Silva LE, de Souza LR, de Oliveira AG. Beta-lactams susceptibility testing of penicillin-resistant, ampicillin-susceptible Enterococcus faecalis isolates: a comparative assessment of Etest and disk diffusion methods against broth dilution. Ann Clin Microbiol Antimicrob. 2020;19(1):1-5. doi: 10.1186/s12941-020-00386-8
- Adhikari L. High-level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J Glob Infect Dis. 2010; 2(3): 231-235. doi: 10.4103/0974-777X.68534
- Kaushik SN, Scoffield J, Andukuri A, Alexander GC, Walker T, Kim S, et al. Evaluation of ciprofloxacin and metronidazole encapsulated biomimetic nanomatrix gel on Enterococcus faecalis and Treponema denticola. Biomater Res. 2015;19(1):1-10. doi: 10.1186/s40824-015-0032-4
- Sood S, Malhotra M, Das BK, Kapil A. Enterococcal infections & antimicrobial resistance. Indian J Med Res. 2008;128(2):111-121.
- Wang Y, Xiong Y, Wang Z, Zheng J, Xu G, Deng Q, et al. Comparison of solithromycin with erythromycin in Enterococcus faecalis and Enterococcus faecium from China: antibacterial activity, clonality, resistance mechanism, and inhibition of biofilm formation. J Antibiot. 2021;74(2):143-151. doi: 10.1038/s41429-020-00374-2
- Rafii F, Wynne R, Heinze TM, Paine DD. Mechanism of metronidazole-resistance by isolates of nitroreductase-producing Enterococcus gallinarum and Enterococcus casseliflavus from the human intestinal tract. FEMS microbiology letters. 2003;225(2):195-200. doi: 10.1016/S0378-1097(03)00513-5
- Bhat KG, Paul C, Bhat MG. High level aminoglycoside resistance in enterococci isolated from hospitalized patients. Ind J Med Res. 1997;105:198-199.
- Conceição N, de Oliveira CD, da Silva LE, de Souza LR, de Oliveira AG. Ampicillin susceptibility can predict in vitro susceptibility of penicillin-resistant, ampicillin-susceptible Enterococcus faecalis isolates to amoxicillin but not to imipenem and piperacillin. J Clin Microbiol. 2012;50(11):3729-3731. doi: 10.1128/JCM.01246-12
- Magaldi S, Mata-Essayag S, De Capriles CH, Pérez C, Colella MT, Olaizola C, et al. Well diffusion for antifungal susceptibility testing. Int J Infect Dis. 2004;8(1):39-45. doi: 10.1016/j.ijid.2003.03.002
- Garrod LP, Waterworth PM. A study of antibiotic sensitivity testing with proposals for simple uniform methods. J Clin Pathol. 1971;24(9):779-789. doi: 10.1136/jcp.24.9.779
- Brives C, Pourraz J. Phage therapy as a potential solution in the fight against AMR: obstacles and possible futures. Palgrave Commun. 2020;6(1):1-11. doi: 10.1057/s41599-020-0478-4
- Kadouri DE, To K, Shanks RM, Doi Y. Predatory bacteria: a potential ally against multidrug-resistant Gram-negative pathogens. PLoS One. 2013;8(5):1-4. doi: 10.1371/journal.pone.0063397
- Benítez-Chao DF, León-Buitimea A, Lerma-Escalera JA, Morones-Ramírez JR. Bacteriocins: An overview of antimicrobial, toxicity, and biosafety assessment by in vivo Front Microbiol. 2021;12:1-18. doi: 10.3389/fmicb.2021.630695
- Antimicrobial susceptibility testing [Internet]. EUCAST disk diffusion method; 2022. [cited Mar 16, 2022]. Available from: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/
- Brown DF, Kothari D. Comparison of antibiotic discs from different sources. J Clin Pathol. 1975;28(10):779-783. doi: 10.1136/jcp.28.10.779
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
