By Nausheen Henna1,2,3, Faiza Shafqat4, Manqoosh Ur Rehman5, Shahzad Ahmed Fakhr6, Sameer Anjum1, Samra Sameen7, Abdul Hannan Nagi3
AFFLIATIONS:
- Department of Pathology, RAK Medical and Health Sciences University, RAK, UAE.
- RAK Hospital, RAK, UAE.
- Department of Pathology, University of Health Sciences, Pakistan.
- Department of Pathology, Bakhtawar Amin Medical and Dental College, Pakistan.
- Department of Orthopedic and Trauma Surgery, Multan Medical and Dental College, Ibn-e-Siena Hospital and Research Institute, Pakistan.
- Department of Anesthesia, Tawam Hospital, Al-Ain, UAE.
- Department of Pathology, Services Institute of Medical Sciences, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-2/002
ORCID iD: 0000-0002-4347-9205
How to cite: Henna N, Shafqat F, Rehman MU, Fakhr SA, Anjum S, Sameen S, et al. Clinicopathological Characteristics of Molecularly Classified Groups of Invasive Ductal Breast Carcinoma in Pakistani Women. Pak J Med Dent. 2022;11(2): 3-8. doi: 10.36283/PJMD11-2/002
Background: The incidence of breast cancer warrants special consideration and understanding of disease demography. The study aimed to determine the clinicopathological characteristics of molecularly classified groups of invasive ductal breast carcinoma in Pakistani women.
Methods: Patients (n=83) undergoing modified radical mastectomy with primary microscopically proven invasive ductal carcinoma were recruited from two tertiary care hospitals, Lahore, Pakistan. Grossing, reporting and biomarker testing was performed as per the College of American Pathologists (CAP) protocols. Chi-square was applied to observe associations between variables. A p-value <0.05 was considered statistically significant.
Results: The mean age in years (mean ± SD) of the patients was 49.3 ±10.98, 50.9 ± 15.4, 50.6 ± 10.9 and 44.6 ± 8.2 having breast carcinoma of luminal A (37.2%), Luminal B (12%), HER2-enriched (20.5%) and triple-negative group (TN) (30.1%), respectively. Nodal involvement was 24(29%), 5(6%), 13(16%) and 17(20.5%) in all groups. Among four groups of breast carcinoma histological grade I was observed as 2.4%, 1.2%, 1.2%, 0%, grade II was recorded as 20.5%, 4.8%, 6 % and 7.2% and grade III as 14.5%, 6%, 13.3% and 22.9%, respectively. Luminal A patients were more in T2 stage whereas more TN patients belonged to T4 stage (p-value = 0.001). A statistically significant association was observed between T2 tumor stage with grade II (p-value = 0.003).
Conclusion: Patients with Luminal A and triple-negative (TN) characteristics were the predominant molecular subtypes. TN patients presented at an earlier age and higher stage compared to other groups whereas, Luminal A profile patients were at the lower tumor stage.
Keywords: Estrogen Nuclear Receptor; Mammary Ductal Carcinoma; Breast; Progesterone; Triple Negative Breast Cancer.
In Pakistan, nearly one in nine female patients has lifetime risk of developing breast cancer1. It is 2.5 times higher incidence in Pakistan than that of neighboring countries and high when compared to Western population, with male to female ratio of 1: 16 2. Statistics shows that there is an alarming rise in breast carcinoma in Pakistan. In terms of death, 63% in developing countries and 37% in developed countries3. As per Global cancer statistics 2020, female breast cancer has become the most commonly diagnosed carcinoma in year 2020 (11.7%) and fourth cause of death from cancer overall and 15.5% mortality among females worldwide4.
Many pathogenetic molecular mechanisms are involved, which correlate with different clinical behaviors. This evolving phenotypic diversity has affected the diagnosis and prognosis of breast cancer. Histological classification gives insufficient prognostic information and biological behavior of the tumor and so do not fully correlate to clinical course and outcome. The biological heterogeneity of tumors continues to be a problem because only a subset of patients with a particular type of tumor will benefit or respond to targeted treatments. Modern molecular test is considered superior to old-fashioned morphology5. Since there is variable treatment response among patients, the objective of the study is to observe the clinicopathological characteristics among different molecular groups.
This was a prospective study comprised of 83 modified radical mastectomy specimens, microscopically confirmed primary invasive ductal carcinoma patients from two tertiary care hospitals of Lahore (Mayo Hospital and Shalamar Hospital) after informed consent. The cases who received neoadjuvant therapy were excluded. Grossing and reporting was performed as per CAP protocol for the “examination of specimens from patients with invasive carcinoma of breast”, version InvasiveBreast 4.1.0.0, 2018. Hormone receptors and HER2neu scoring was performed using CAP “Reporting results of biomarker testing of specimens from patients with carcinoma of the breast”, version: BreastBiomarkers 1.2.0.0, 2018.
Using molecular classification with the help of immunohistochemistry, breast carcinoma has been divided into following groups as: Luminal A (ER positive, PR positive, HER2/neu negative), Luminal B (ER positive, PR positive, HER2/neu positive), Non-luminal/ HER2 –enriched (ER negative, PR negative, HER2/neu positive) and Triple negative/Basal type (ER negative, PR negative, HER2/neu negative) 3,6. The data was entered and analysed using IBM SPSS version 27. Frequencies and percentages were reported for qualitative variables. Pearson Chi-square was applied to observe associations between qualitative variables. A p-value of less than 0.05 was considered as statistically significant.
Eighty-three patients with invasive ductal carcinoma were recruited in this study with mean age ± SD of 48.8 ± 10.9 years. The mean age ± SD in Luminal A group was 49.3 ±10.98 years, in luminal B 50.9 ± 15.4, in HER2neu Enriched 50.6 ± 10.9 and that of triple negative was 44.6 ± 8.2 years. 69.9% of patients were below 50 years of age (Table 1).
Table 1: Characteristics of age, laterality, nodal stage, tumor stage, Nottingham Grade of different molecular groups.
| Parameters | Characteristics | Luminal A
n (%) |
Luminal B
n (%) |
HER2neu enriched
n (%) |
Triple negative
n (%) |
Pearson Chi square | p-Value |
|
Age |
<50 years | 18 (21.7) | 7 (8.4) | 11 (13.3) | 22 (26.5) | 6.172 | 0.104 |
| >50 years | 13 (15.7) | 3 (3.6) | 6 (7.2) | 3 (3.6) | |||
|
Laterality |
Left | 18 (21.7) | 6 (7.2) | 11 (13.3) | 16 (19.3) | 0.304 | 0.959 |
| Right | 13 (15.7) | 4 (4.8) | 6 (7.2) | 9 (10.8) | |||
|
Nodal Stage |
No | 7 (8.4) | 5 (6) | 4 (4.8) | 8 (9.6) | 6.295 | 0.710 |
| N1 | 10 (12) | 2 (2.4) | 9 (10.8) | 8 (9.6) | |||
| N2 | 10 (12) | 2 (2.4) | 3 (3.6) | 7 (8.4) | |||
| N3 | 4 (4.8) | 1 (1.2) | 1 (1.2) | 2 (2.4) | |||
|
Tumor Stage |
T2 | 18 (21.7) | 7 (8.4) | 8 (9.6) | 7 (8.4) | 23.244 | 0.001 |
| T3 | 11 (13.3) | 3 (3.6) | 7 (8.4) | 5 (6) | |||
| T4 | 2 (2.4) | 0 | 2 (2.4) | 13 (15.7) | |||
|
Nottingham Grade |
I | 2 (2.4) | 1 (1.2) | 1 (1.2) | 0 | 9.541 | 0.145 |
| II | 17 (20.5) | 4 (4.8) | 5 (6) | 6 (7.2) | |||
| III | 12 (14.5) | 5 (6) | 11 (13.3) | 19 (22.9) |
There were 37.2% of patients who expressed luminal A profile, 12% of patients showed luminal B profile, HER2-enriched expression was present in 20.5% and 30.1% patients were triple negative. Tumor size was more than 2cm in most of the cases (80%), whereas, it was less than/equal to 2cm in only 3.6% of cases (Table 2). Lymph node metastasis was identified in 59 (71.1%) patients, whereas, it was negative in 24 (29%) (Table 2). It was observed in 24 (28.9%) in Luminal A group, 5 (6.2%) in luminal B group, 13 (15.7%) in HER2-enriched group and 17 (20.5%) in triple negative patients.
Table 2: Molecular groups characteristics with reference to tumor size and lymph node metastasis.
| Characteristics | Luminal A
n (%) |
Luminal B
n (%) |
HER-2 positive
n (%) |
Triple negative n (%) |
Total
n (%) |
| Age ≤ 50 | 18 (21.7) | 7 (8.4) | 11 (13.3) | 22 (26.5) | 58 (70) |
| > 50 | 13 (15.7) | 3 (3.6) | 6 (7.2) | 3 (3.6) | 25 (30) |
| Tumor size
≤ 2 > 2- ≤ 5 > 5 |
1 (1.2) 19 (23) 11 (13.3) |
1 (1.2) 7 (8.4) 2 (2.4) |
0 8 (9.6) 9 (10.8) |
1 (1.2) 6 (7.2) 18 (21.7) |
3 (3.6) 40 (48.2) 40 (48.2) |
| Lymph Node Metastasis | |||||
| Negative |
7 (8.4) |
5 (6) |
4 (4.8) |
8 (9.6) |
24 (29) |
| Positive
1-3 >4 |
24 (29)
10 (12) 14 (17) |
5 (6)
2 (2.4) 3 (3.6) |
13 (16)
10 (12) 3 (3.6) |
17 (20.5)
9 (10.8) 8 (9.6) |
59 (71.1)
31 (37.3) 28 (33.7) |
More patients in Luminal A, B and HER2-enriched group were in T2 stage, whereas, triple negative patients presented at T4 stage i.e., 13 (15.7%) among all groups. In the present study, Nottingham grade III was observed in 56.6% of patients, grade II in 38.6% and grade 1 in 4.8% of patients. Triple negative patients showed highest percentage of grade III, Luminal A showed highest in grade II and grade I was seen in 2 patients in Luminal A, 1 each patient in Luminal B and HER2- enriched groups. Statistically significant association was observed in T2 stage in Luminal A (21.7%) and T4 stage in Triple negative patients (15.7%). Statistical analysis shows significant association of higher tumor stage was with higher Nottingham grade (Figure 1). Microphotograph shows the invasive ductal carcinoma along with biomarkers immunohistochemistry (Figure 2).
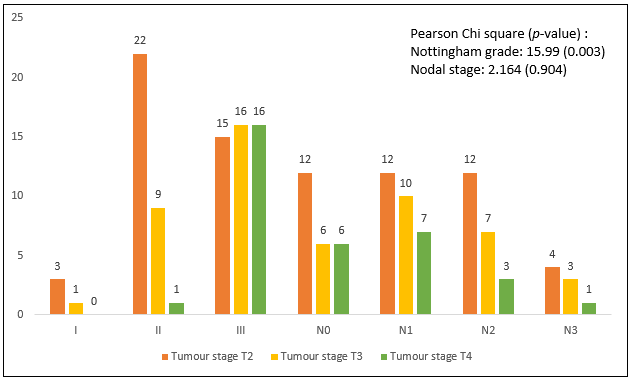
Figure 1: Association of tumor stage with Nottingham Grade and nodal stage. Pearson Chi square value along with p-value for Nottingham Histological Grade and nodal stage has been mentioned on right top.
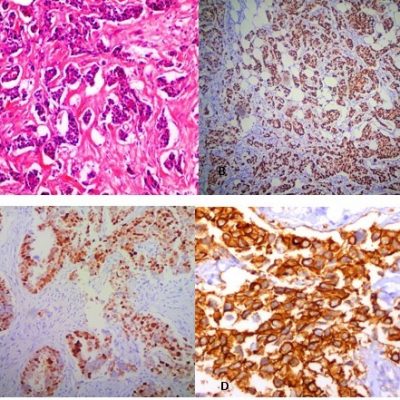
Figure 2: A. Microphotograph shows moderately differentiated invasive ductal carcinoma with extensive desmoplastic reaction (100X). B: Microphotograph shows estrogen receptor with Allred Score of 5+ 3= 8, considered as positive (40x). C: Microphotograph shows progesterone receptor with Allred Score of 5+3=8 considered as positive (200x). D: Microphotograph shows a case where HER2neu was reported as +3 (400x).
In current era of advancement, pathologists are now considered as “diagnostic oncologists” and play critical role as clinical consultants on the biology of disease. During last few years, deep insights of the molecular information has transformed the understanding of histologic diversity of breast cancers and redirected the way of management.
In the present study, more patients turned out to be in luminal A group followed by triple negative, HER2neu enriched and luminal B in descending order. An Indian study showed Luminal A profiling in 60%, Luminal B in 3.3%, HER2 positive in 10% and Triple negative in 26% of patients7. Badar et al. reported 3.7% in Luminal A, 37.3% in Luminal B, 10.9% in HER2-enriched) and 16.6% in triple negative in their study group8. A study conducted in Abbottabad found 28.33% in Luminal A group, 25% in Luminal B, HER2 enriched group comprised of 30% and triple negative patients were 10% 9. These findings show that patients have heterogeneous molecular features in same population, which warrants the demand of personalized, individualized targeted therapy.
A study conducted in Saudi Arabia, which included ductal, lobular and other carcinomas quoted luminal A as most prevalent group and HER2 positive was least10. However, the current study groups included only ductal carcinoma with Luminal A being most common followed by triple negative, HER2neu enriched and Luminal B groups. Rosa conducted research in Florida, studied patients comprised of 40% Luminal A, 20% Luminal B, HER2 enriched 20-30% and Basal type/Triple negative 15% 5. Reddy et al. reported Luminal A in 36.1%, luminal B in 3.7%, Her2/neu in 28.7% and triple negative in 31.5% 6. An Iranian population-based study quoted Luminal A in 54%, luminal B in 22%, HER2-enriched in 14% and triple negative in 10% 11.
Millar et al., Australian research had patients 79.1% Luminal A, 4.6%, Luminal B 12. Chinese study group had patients who belong to Luminal A (32.8%), Luminal B (27.9%) 9.9%, Her2-enriched group (13.3%) and Triple negative group comprised of 26.7% of patients13. Literature search shows that Luminal A is most prevalent group in Oman, Poland, China, Peru, Tunisia, USA, Riyadh and Jeddah10, 14-19. An Iranian study stated that Luminal B was most common (43.73%) in their study group followed by Luminal A (27.97%), HER2 Enriched (20.9%) and triple negative (7.4%) in descending order 20.
The observation of HER2-enriched group of present study are in homogenous with Shaukat Khanum Hospital, Lahore, Pakistan study findings conducted by Badar et al., where they observed positivity in 24.6%, negative in 53.9% 8. Song et al., reported positivity 23.2 % and Inwald et al., observed in 18.2% of patients21,22. Yamamoto et al. observed HER2Neu overexpression in 6%, equivocal in 20%, negative in 73% of patients23 whereas, Choi et al. findings revealed 12.4% positivity of HER2 neu24.
Akbar et al., mentioned that 10 (16.7%) and 7 (11.7%) patients belonged to histological grade II and III in luminal A group, 12 (20%) and 3 (5%) patients belonged to grade II and III in luminal group B, single case (1.6%) belonged to grade I, 9 (15%) and 8 (13.1%) patients belonged to histological grade II and III in HER2 neu enriched group, and 2 (3.3%) and 8 (13.1%) patients belonged to grade II and III in triple negative group9. These observations are comparable to the current study. The research results are also in concordance with the results of Bennis et al., where the Nottingham Grade II was most common in Luminal A and grade III in triple negative group25.
An African population-based study conducted in 2020 reported that their most patients in Luminal A group presented at T2 stage (46.6%), Luminal B patients were also most common in T2 stage, triple negative patients at T4 stage and all the patients with Her2 positivity belonged to T4 stage26. These results are comparable with the observations made in this study, as T2 (21.7% and 8.4%) stage in Luminal A and Luminal B group respectively, T4 stage was more prevalent in triple negative patients (15.7%). However, research by Mohammad could not find any significant association with tumor stage, which is in contrary to the present study20.
Patient with Luminal A and triple negative characteristic were the predominant molecular subtypes. Triple negative patients are presenting at an earlier age and higher stage compared to other groups. Luminal A profile patients presented at lower tumor stage, while triple negative patients presented at higher stage. There is a wide variability observed in clinicopathological characteristics of patients among molecular groups, which reflects the outcome differences of treatment among Pakistani women.
We would like to thank the Hospital and Pathology Department Administration of Mayo Hospital and Shalamar Hospital for facilitation and smooth conduction of research, with special thanks to Mr. Shafiq of Pathology Laboratory, Mayo Hospital, Lahore, Pakistan.
The authors declare no conflict of interest.
The study was approved as part of Ph.D. research by Advanced Studies and Research Board, University of Health Science, Pakistan (# UHS/Education/126-16/215).
The research was funded by the University of Health Sciences, Lahore, Pakistan.
Informed consent was taken from patients. Patient identity was not disclosed at any point during the research.
NH designed the project, data processing, collection, analysis, manuscript drafting; FS, MR, SAF performed analysis and interpretation of results; SS performed sample collection and critical revision of article; SA performed the laboratory work; AHN supervised the whole project and critical revision.
- Zaheer S, Shah N, Maqbool SA, Soomro NM. Estimates of past and future time trends in age-specific breast cancer incidence among women in Karachi, Pakistan: 2004–2025. BMC Public Health. 2019;19(1):1-9. doi: 10.1186/s12889-019-7330-z
- Asif HM, Sultana S, Akhtar N, Rehman JU, Rehman RU. Prevalence, risk factors and disease knowledge of breast cancer in Pakistan. Asian Pac J Cancer Prev. 2014;15(11):4411-4416. doi: 10.7314/apjcp.2014.15.11.4411
- Bandala C, De la Garza-Montano P, Cortes-Algara A, Cruz-Lopez J, Dominguez-Rubio R, Gonzalez-Lopez NJ, et al. Association of histopathological markers with clinico-pathological factors in Mexican women with breast cancer. Asian Pac J Cancer Prev. 2016;16(18):8397-8403. doi: 10.7314/apjcp.2015.16.18.8397
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660
- Rosa M. Advances in the molecular analysis of breast cancer: pathway toward personalized medicine. Cancer Control. 2015;22(2):211-219. doi: 10.1177/107327481502200213
- Reddy GM, Suresh PK, Pai RR. Clinicopathological features of triple negative breast carcinoma. J Clin Diagn Res. 2017; 11(1): EC05-EC08. doi: 10.7860/JCDR/2017/21452.9187
- Gupta P, Rai NN, Agarwal L, Namdev S. Comparison of molecular subtypes of carcinoma of the breast in two different age groups: a single institution experience. Cureus. 2018;10(6):1-10. doi:10.7759/cureus.2834
- Badar F, Mahmood S, Faraz R, Yousaf A, Quader AU, Asif H. Epidemiology of breast cancer at the Shaukat Khanum memorial cancer hospital and research center, Lahore, Pakistan. J Coll Physicians Surg Pak. 2015;25(10):738-742.
- Akbar M, Akbar K, Naveed D. Frequency and correlation of molecular subtypes of breast cancer with clinicopathological features. J Ayub Med Coll Abbottabad. 2014;26(3):290-293.
- Al-Thoubaity FK. Molecular classification of breast cancer: A retrospective cohort study. Ann Med Surg. 2020;49:44-48. doi: 10.1016/j.amsu.2019.11.021
- Moazzezy N, Ebrahimi F, Sisakht MM, Yahyazadeh H, Bouzari S, Oloomi M. Relationship between erb-B2 mRNA expression in blood and tissue of invasive ductal carcinoma breast cancer patients and clinicopathological characteristics of the tumors. Asian Pac J Cancer Prev. 2016;17(1):249-254. doi: 10.7314/apjcp.2016.17.1.249
- Millar EK, Graham PH, McNeil CM, Browne L, O’Toole SA, Boulghourjian A, et al. Prediction of outcome of early ER+ breast cancer is improved using a biomarker panel, which includes Ki-67 and p53. Br J Cancer. 2011;105(2):272-280. doi: 10.1038/bjc.2011.228
- Liu X, Guan Y, Zhang W, Liu S, Liu J, Wang L, et al. Predictors of recurrence in breast cancer subtypes with negative lymph node in a Chinese population. Int J Clin Exp Pathol. 2014; 7(6): 3202-3212.
- Mehdi I, Monem AA, Al Bahrani B, Ramadhan FA. Breast cancer molecular subtypes in oman: correlation with age, histology, and stage distribution-analysis of 542 cases. Gulf J Oncolog. 2014;1(15):38-48.
- Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439-443. doi: 10.1158/1055-9965.EPI-06-0806
- Cheng HT, Huang T, Wang W, Yue JQ, Shen N, Guo H, et al. Clinicopathological features of breast cancer with different molecular subtypes in Chinese women. J Huazhong Univ Sci Technolog Med Sci. 2013;33(1):117-121. doi: 10.1007/s11596-013-1082-2
- Vallejos CS, Gómez HL, Cruz WR, Pinto JA, Dyer RR, Velarde R, et al. Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a peruvian hospital database. Clin Breast Cancer. 2010;10(4):294-300. doi: 10.3816/CBC.2010.n.038
- Fourati A, Boussen H, El May MV, Goucha A, Dabbabi B, Gamoudi A, Sfar R, Rahal K, El May A, Ben Abdallah M. Descriptive analysis of molecular subtypes in T unisian breast cancer. Asia Pac J Clin Oncol. 2014;10(2):e69-e74. doi: 10.1111/ajco.12034
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-2502.
- Mohammed AA. The clinical behavior of different molecular subtypes of breast cancer. Cancer Treat Res Commun. 2021;29:1-6. doi: 10.1016/j.ctarc.2021.100469
- Song N, Choi JY, Sung H, Jeon S, Chung S, Song M, et al. Tumor subtype-specific associations of hormone-related reproductive factors on breast cancer survival. PLoS One. 2015;10(4):1-15. doi: doi.org/10.1371/journal.pone.0123994
- Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539-552. doi: 10.1007/s10549-013-2560-8
- Yamamoto M, Hosoda M, Nakano K, Jia S, Hatanaka KC, Takakuwa E, et al. p53 accumulation is a strong predictor of recurrence in estrogen receptor‐positive breast cancer patients treated with aromatase inhibitors. Cancer Sci. 2014;105(1):81-88. doi: 10.1111/cas.12302
- Choi YJ, Shin YD, Song YJ. Comparison of ipsilateral breast tumor recurrence after breast-conserving surgery between ductal carcinoma in situ and invasive breast cancer. World J Surg Oncol. 2016;14(1):1-8. doi: 10.1186/s12957-016-0885-6
- Bennis S, Abbass F, Akasbi Y, Znati K, Joutei KA, El Mesbahi O, et al. Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco: retrospective study. BMC Res Notes. 2012;5(1):1-8. doi: 10.1186/1756-0500-5-436
- Adani-Ifè A, Amégbor K, Doh K, Darré T. Breast cancer in togolese women: immunohistochemistry subtypes. BMC Womens Health. 2020 Dec;20(1):1-7. doi: 10.1186/s12905-020-01130-2
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
