By Bushra Wasim1, Marvi Farooqui2, Khalid M. Khan3, Nighat Kafil4
AFFLIATIONS:
- Department of Anatomy, Ziauddin University, Karachi, Pakistan.
- Ziauddin University, Karachi, Pakistan.
- Department of Anatomy, University of Kuwait, Kuwait.
- Department of Pharmacology, Mohammad Medical College Mirpurkhas, Sindh, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-1/005
How to cite: Wasim B, Farooqui M, Khan KM, Kafil N. Assessment of 1 α-Hydroxylase in Vitamin D- Deficient Premenopausal Pakistani Females. Pak J Med Dent. 2022;11(1): 25-31. doi: 10.36283/PJMD11-1/005
Background: 25-hydroxyvitamin D-1-α-hydroxylase is a key enzyme for conversion of 25-(OH)-D to 1,25-(OH)2-D and is extra renally produced in various tissues. The objective of the study was to determine the levels of enzyme 1-α-hydroxylase in response to supplementation with vitamin D in premenopausal women.
Methods: This single-arm pre-post interventional study, included premenopausal females (n=82) and their 1-α hydroxylase levels were measured from peripheral blood. Vitamin D supplements were given and vitamin D levels and α-1 hydroxylase levels were measured through ELISA. The serum vitamin D levels below 30ng/ml (75nmol/L), were considered vitamin D deficient. The correlation between serum vitamin D levels and 1-α-OHase levels was determined using Spearman’s Rank Correlation test, Wessa P Spearman Rank Correlation in Free Statistics Software. Relationships between variables were done through paired t-test and p<0.05 was considered significant.
Results: The levels of 1-α (OH)ase varied in response to supplementation, with most values observed between 10-20ng/ml. The average increase in serum vitamin D and 1-α-(OH)ase levels was 9.49ng/mL and 4.66ng/mL respectively. In total 50(60.9%) samples revealed a decrease in 1-α (OH)ase levels, and 32(39.1%) samples increased. Data analysis showed t (84) = 1.214, p=0.228 for serum 1- α (OH)ase. For post-supplementation the results showed no association between (rs = 0.04982, p = 0.65671) the two variables.
Conclusion: Serum levels of 1-α-hydroxylase in premenopausal Pakistani females indicated variation when vitamin D was supplemented, highlighting a non-linear relationship. This shows involvement of other unknown factors in Vitamin D metabolism, which requires to be investigated in future research.
Keywords: Biopsy; Inflammation; Hydroxylase.
Research on vitamin D and its derivatives has reached its zenith in the past few years. Studies show 50% of the human population worldwide is affected by a deficiency of this vitamin1. There is a higher preponderance in countries that lie on or near the equator, as countries receiving stronger ultraviolet B waves (UVB) throughout the year are home to individuals with more melanin. This effect is protective from the standpoint of skin cancer prevention since it reduces the skin’s ability to absorb UVB, but the other side of the coin is a decrease in Vitamin D production2,3. For such populations, the only way to reach the recommended daily allowance (RDA) is to incorporate vitamin D into daily foods and supplements4. The authors of this study reside in a country where the prevalence of vitamin D deficiency across various cities is ≈84%, despite adequate exposure to sun5. Vitamin D Deficiency has been defined as serum/plasma 25(OH)D levels falling below 30ng/ml (75nmol/L), with levels below 20ng/ml (or 50nmol/L) as the “cut-off” for abnormal biochemical functioning. Local data done from 1998 indicates 35% of women were deficient6. A repeat survey done after 10 years on different sample groups showed a stark increase in deficiency of up to 95% 7. The recommended level issued by the Institute of Medicine for adult RDA is between 10-20 micrograms a day, with recommendations for a slightly higher RDA for those with darker skin8. For severely deficient patients, once-a-day dosing of 40 micrograms of calcidiol has been suggested1.
The precise link between vitamin D metabolism and the effect of supplementary dosing is still unclear, specifically, there are very few studies relating 1-α-hydroxylase with vitamin D supplementation. Of the existing research, a study done by Stubbs et al. on monocytic 1-α-hydroxylase by flow cytometry showed a decrease in the intracellular levels of the enzyme after supplementation with cholecalciferol9.
25-hydroxyvitamin D-1-α-hydroxylase is the key enzyme for the conversion of 25-(OH)-D to 1,25-(OH)2-D. It is found in renal as well as extra-renal cells on the membranes of mitochondria (CYP27B1)10. The extra-renal role of 1-α hydroxylase in cancers of the breast, colon, prostate and parathyroid has been investigated over the years. Current literature provides information on levels of human 1-α(OH)ase via real-time polymerase chain reaction (qRT-PCR), Western blot analyses and immunohistochemical methods10; however, as far as the publishing of this paper, we are not aware of any studies done via ELISA comparing levels of 1- α-hydroxylase with vitamin D supplementation11-15. We hypothesized that vitamin D supplementation causes a linearly proportional decrease in 1-αhydroxylase levels of white blood cells (WBCs). Therefore, the purpose of this study was to associate levels of serum 1-α(OH)ase with supplementation given in premenopausal women.
In this single-arm pre-post interventional study, we took a group of premenopausal females (n=82) and measured their serum vitamin D levels and their 1-α hydroxylase levels from peripheral blood. Sample selection was conducted via convenience sampling based on the patients who came to General Surgery Outpatient Department (OPD). Approval was taken from the Ethics Review Committee Ziauddin University (Ref no. 0330512BWANT) and informed consents were taken from the participating patients.
The criteria for inclusion were females under the age of 40 years with serum 25-OH-D levels of less than 20ng/ml, history of breast lump or cancer, and family history of breast or other cancers. Criteria for exclusion were individuals who were pregnant or lactating, unknown menopausal status and taking oral contraceptives. Other variables taken into consideration were the number of pregnancies, whether the females had breastfed their children and for how long, age of menarche, and levels of serum calcium.
Vitamin D supplements were given for a specified time and repeated samples from their blood, measuring both variables (vitamin D levels and 1-α hydroxylase). Patients with Vitamin D levels of <10ng/ml were given 5 injections of Vitamin D3 (Cholecalciferol) 600,000 IU I/M weekly over 5 weeks, followed by oral vitamin D3 tablets 50,000IU weekly for 30 weeks. For patients with serum vitamin D levels between 10-20ng/ml, oral supplementation of Cholecalciferol 50,000 IU was prescribed1,8. Samples of venous blood were collected in Ethylenediaminetetraacetic acid (EDTA) test tubes to prevent coagulation. The tubes were then placed in a centrifuge (at 2000-3000 RPM) for approximately 20 minutes, and the supernatants were carefully collected. To isolate 1-α(OH)ase via liquid biopsy, we used enzyme-linked immune sorbent assay (ELISA) based on biotin double antibody sandwich technology to separate the enzyme according to standard protocol. The assay range for the kit was 0.2ng/ml→60ng/ml, and the sensitivity was 0.1ng/ml. Data were entered into Microsoft Excel and was analyzed using IBM SPSS Statistics software v.20. Relationships between variables were done through paired t-test and p<0.05 was considered significant. Correlation between serum vitamin D levels and 1-α-OHase levels was done using Spearman’s Rank Correlation test, using Wessa P Spearman Rank Correlation (v1.0.3) in Free Statistics Software (v1.2.1).
A total of 294 females were evaluated during the intervention, out of which 82 (27.9%) were included in this study. The mean age of the participants was 30.03, and all were pre-menopausal. There was a comparable distribution of ages in the study population, with most lying in the 20–25-year age range 25 (30.5%) and others were married (73.2%). Most of the population were graduates (31.7%). A little over half the participants belonged to Muhajir ethnicity (52.4%). Most women (41.5%) reached menarche at 13 years of age. Only 25 (30.5%) of the women were nulliparous, i.e., had not had any children – out of the other 57 (69.5%), a majority had two offspring (29.8%). The mother’s age at the first child was between 21 to 30 years in most cases (49.1%), and the mother’s age at last or most recent child was also between 21 and 30 years in most cases (63.2%). A vast majority of women breastfed their child (84.2%), with 60.4% breastfeeding for a period of up to 24 months as shown in Table 1a.
Table 1a. Socio-demographic characteristics of study participants.
| Variables Frequency (%) | ||||||
| Age (years) (Mean+SD) | 30.03+0.32 | |||||
| Age Range (years) | 20-25 | 26-30 | 31-35 | 36-40 | ||
| 25 (30.5) | 22 (26.8) | 15 (18.3) | 20 (24.4) | |||
| Marriage Status | Unmarried | Married | Widowed | Divorced | ||
| 10 (12.2) | 60 (73.2) | 2 (2.4) | 2 (2.4) | |||
| Level of Education | Primary | Secondary | Matric | Inter | Graduate | Unknown |
| 4 (4.9) | 23 (28.0) | 3 (3.7) | 6 (7.3) | 26 (31.7) | 20 (24.4) | |
| Ethnicity | Sindhi | Punjabi | Pathan | Muhajir | Balochi | Other |
| 1 (1.2) | 3 (3.7) | 12 (14.6) | 43 (52.4) | 3 (3.7) | 20 (23.7) | |
| Age at Menarche (years) | 11 | 12 | 13 | 14 | 15 | Could not remember |
| 3 (3.7) | 23 (28.0) | 34 (41.5) | 15 (18.3) | 1 (1.2) | 6 (7.3) | |
| Age at First Child (years) | 13-20 | 21-30 | 31-40 | Did not remember | ||
| 25 (43.9) | 28 (49.1) | 2 (3.5) | 3 (5.3) | |||
| Age at Last Child (years) | 18-20 | 21-30 | 31-40 | Did not remember | ||
| 3 (5.3) | 36 (63.2) | 7 (12.3) | 11 (19.3) | |||
| Duration of Breastfeeding (months) (n=48) | Less than 1 month | 1 to 6 | 7 to 12 | 18 | 24 | 36 |
| 2 (4.2) | 7 (14.6) | 5 (10.4) | 3 (6.3) | 29 (60.4) | 2 (4.2) | |
We also found participants who had a positive history of breast cancer (22.0%). Family history of any cancer was positive in 23 (28.0%) of the women. Previous breast-related complaints included breast lump (37.8%), breast pain (39.0%) and history of lumpectomy (34.1%). Out of 82 patients selected in the study, 21 (25.6%) presented to the clinic with a breast lump, 19 (23.2%) presented with pain, 1(1.2%) presented with itching and 42 (51.2) came with no symptoms at all. On examination, 5(6.1%) had nipple discharge, 10(12.2%) had a palpable mass or lump, and 4(4.9%) had an axillary mass or masses (Table 1b.)
Table 1b. Summary of past medical history and examination findings of study participants.
| Variables | Frequency (%) |
| Personal History of Breast Cancer | 18 (22.0) |
| Family History of Any Cancer | 23 (28.0) |
| Past Complaints of Breast Lump | 31 (37.8) |
| Complaints of Breast Pain | 32 (39.0) |
| History of Lumpectomy | 28 (34.1) |
| Presenting Symptoms
Lump Pain Itching No symptoms |
21 (25.6)19 (23.2)
1 (1.2) 42 (51.2) |
| Nipple Discharge on Examination | 5 (6.1) |
| Mass or Lump on Examination | 10 (12.2) |
| Axillary Mass on Examination | 4 (4.9) |
All the participants were considered vitamin D deficient, with baseline serum vitamin D levels below 10 ng/mL in 74.4%, between 10 to 20ng/mL in 19.5%, and between 20 to 30ng/mL in 6.1% of the participants. For all 82 participants, the levels of serum vitamin D had raised above 30ng/ml after supplementation, as shown in Table 3.
Table 3: Serum levels of vitamin D before and after supplementation.
| Intervention | Serum Levels Vitamin D (ng/mL) | Total | |||
| <10 | 10 to 20 | 20 to 30 | >30 | ||
| Pre supplement (n) | 61 (74.4) | 16 (19.5) | 5 (6.1) | 0 | 82 |
| Post supplement (n) | 0 | 0 | 0 | 82 (100) | 82 |
Serum levels of calcium were recorded from baseline samples, averaging at 9.41mg/dL for 82 participants. The average % increase in serum vitamin D and 1-α-(OH)ase levels was 9.49ng/mL and 4.66ng/mL respectively, as shown in Table 4. The levels of 1-α (OH)ase varied in response to supplementation, with most values observed between 10-20ng/ml, regardless of pre-or post-supplementation status. 60.9% of samples revealed a decrease in 1-α (OH)ase levels, and 39.1% of samples revealed an increase in 1-α (OH)ase levels after supplementation, as shown in Table 4. Data analysis showed t (84) = 1.214, p=0.228 for serum 1-α (OH)ase levels.
Table 4: Serum levels of calcium compared against serum vitamin D (pre and post supplementation) and Alpha-1-(OH)ase (pre and post supplementation).
|
|
Serum Levels | p-Value | ||||||
| Serum Calcium(mg/dL) |
Serum Vitamin D
(ng/mL) |
Serum Alpha-1-(Oh)ase
(ng/mL) |
||||||
| Baseline | Pre -supplement | Post -supplement | % Difference | Pre -supplement | Post -supplement | % Difference | ||
| Min | 7 | 3 | 30.1 | 🡩 0.72 | 0.042 | 2 | 🡫 0.96 | 0.228 |
| Max | 11.3 | 29.5 | 150 | 🡩 36.5 | 66.59 | 37.52 | 🡩 361.14 | |
| Avg | 9.41 | 8.76 | 70.22 | 🡩 9.49 | 15.83 | 13.18 | 🡩 4.66 | |
After running Spearman’s correlation test on the pre-supplement variables (Figure 1a) the results showed rs = -0.22048, p = 0.04654 indicating the negative association between the two variables which is statistically significant. For post-supplementation variables, the results showed rs = 0.04982, p = 0.65671 indicating that by normal standards, the association between the two variables would not be considered statistically significant (Figure 1b).
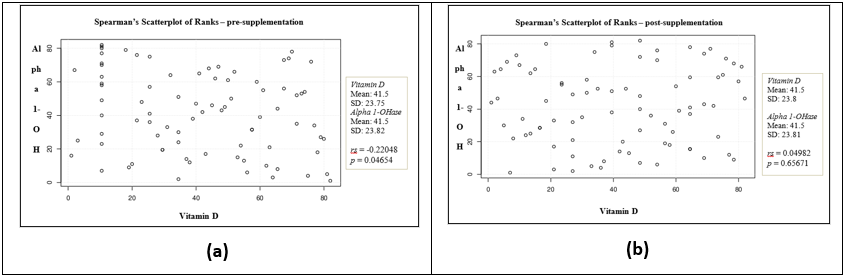
Figure 1a: Spearman’s Scatter plot of ranks – pre-supplementation showing normal standards of the association between the two variables (i.e., serum Vitamin D and serum 1-α hydroxylase) and could be considered statistically significant. 1b: The analysis of Spearman’s scatter plot of ranks – post-supplementation showing exogenous supplementation of Vitamin D which does not show association with the levels of 1-α hydroxylase enzyme in the blood.
These results indicate that there is no linear relationship of the serum levels of 1-α (OH)ase with exogenous supplementation of vitamin D in those individuals who were vitamin D sufficient. However, in individuals who were vitamin D deficient, there were an association of serum 1-α (OH)ase levels with administration of exogenous vitamin D.
The results of this study show that there is not a linearly proportionate relationship between vitamin D supplementation and 1-α hydroxylase levels in serum of premenopausal females coming to a breast clinic, since in some patients’ external vitamin D caused an increase in 1-α hydroxylase activity and others, it caused a decrease. In a study conducted on 1-α hydroxylase knockout mice, it was discovered that the levels of plasma 1, 25-Vitamin D3 remained zero due to the absence of the enzyme despite the knock-out mice being given a feed supplemented with calcium and vitamin D16.
In another study, researchers found increased levels of 24(OH)ase and decreased levels of serum calcitriol causing a decrease in monocyte activation and differentiation into macrophages and dendritic cells17. In our research, levels of 1-α (OH)ase did not fall proportionately in all individuals of the sample population some increased in response to supplementation, regardless of history of breast disease. This unpredictability of the enzyme leads us to question whether there are other factors involved in the cellular metabolism of 1,25-OH-D and whether these factors contribute to the protective effect that vitamin D has in tumorigenesis. Previously conducted randomized control trials on two separate population groups have shown that there is a significant benefit of using vitamin D and calcium in reducing cancer incidence18.
In a 2019 experimental trial, researchers investigated ablated CYP27B1 in mouse models and results showed vitamin D dysregulation, resulting in impairment of anti-tumor properties19. The relationship between Vitamin D Receptor (VDR) and tumor suppression with vitamin D supplementation may well be one of the factors in the equation. When 1,25-OH-D forms complex with its receptor, VDR, it downregulates itself by controlling the expression of CYP27B1 and therefore reducing the rate of conversion of 25-OH-D to 1,25-OH-D. Hence, we can propose the theory that some patients in our study may have reduced expressions of Vitamin D receptor (VDR) which affected the levels of 1-α hydroxylase.
This study was based on the theory that the intracellular dysmetabolism of 1-α hydroxylase from serum affects cells in other tissues, such as breast tissue, by way of an increase in the overall pro-inflammatory cytokines released in the bloodstream20,21. Various biomarkers such as IL-6are in circulation and CD4+ and CD8+ T cells are actively involved in various inflammatory processes22. A study conducted by Calton et al. reported a directly proportional relationship between Vitamin D and Bioenergetic Health Index (BHI). The BHI is a new marker of prognosis for diseased metabolic states such as diabetes and obesity, which are linked with prolonged and/or recurrent inflammation. Calton et al. found that when the vitamin demand was not being met, the levels of BHI also fell – providing more evidence to the theory that a sufficient level of vitamin D can help maintain a decreased inflammation, decreased insulin resistance state23,24.
Similarly, Bikle et al. published a paper that corroborates the strong link between variations in blood cell numbers, inflammatory biomarkers, and seasonal changes, pointing to more evidence of higher pro-inflammatory states in colder weather than in warm weather25. Increases in 25(OH)D in summer months were related to a reduction in systemic inflammation and peripheral blood mononuclear cells (PBMC) bioenergetic profiles and decreased metabolism. Changes were evident in those who had insufficient levels in winter (when the UV levels are generally low). The link between tumorigenesis, pro-inflammatory states and the effects of vitamin D is, therefore, a well-established one. Despite this data, more research is required on whether 1-α (OH)ase has a greater role in maintaining cell homeostasis than we initially thought, and whether it plays a role in the prevention of cancer propagation by reducing inflammation in the body.
There is a variation in the levels of 1-α-hydroxylase expression with vitamin D supplementation with non-linear increases and decreases, pointing to the conclusion that vitamin D alone does not influence 1-α-hydroxylase regardless of the presence or absence of carcinogenic disease. There are other biochemical or genetic factors, yet uninvestigated, that may play a role in vitamin D metabolism in premenopausal Pakistani females, which raises questions into what influences activation and deactivation of 1-α-hydroxylase.
The authors would like to thank the staff and faculty at the MDR Lab and Ziauddin University.
The authors reported no conflict of interest.
The ethics review committee at Ziauddin University (Reference No. 033051BWANT) approved the study. The study complied with ethical principles for medical research involving human participants in line with the Declaration of Helsinki.
Written and verbal informed consent of each patient was taken for this study.
BW formulated the research question and designed the study. MF drafted and prepared the manuscript. BW, KK, and NK were involved in the acquisition of data and statistical analysis, and all authors read and approved the final manuscript.
- Biondi P, Pepe J, Biamonte F, Occhiuto M, Parisi M, Demofonti C, et al. Oral calcidiol is a good form of vitamin D supplementation. Clin Cases Miner Bone Metab. 2017;14(2):207-208. doi: 10.11138/ccmbm/2017.14.1.207
- Young AR, Morgan KA, Ho TW, Ojimba N, Harrison GI, Lawrence KP, et al. Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: A comparison of extreme phenotypes. J Invest Dermatol. 2020;140(7):1418-1426. doi: 10.1016/j.jid.2019.11.019
- Miller WL. Genetic disorders of Vitamin D biosynthesis and degradation. J Steroid Biochem Mol Biol. 2017;165:101-108. doi: 10.1016/j.jsbmb.2016.04.001
- Jamil NA, Yew MH, Noor Hafizah Y, Gray SR, Poh BK, Macdonald HM. Estimated vitamin D synthesis and dietary vitamin D intake among Asians in two distinct geographical locations (Kuala Lumpur, 3 degrees N v. Aberdeen, 57 degrees N) and climates. Public Health Nutr. 2018;21(17):3118-3124. doi: 10.1017/S1368980018002057
- O’Mahony L, Stepien M, Gibney MJ, Nugent AP, Brennan L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients. 2011;3(12):1023-1041. doi: 10.3390/nu3121023
- Zuberi LM, Habib A, Haque N, Jabbar A. Vitamin D Deficiency in ambulatory patients. J Pak Med Assoc. 2008;58(9):482-484.
- Dar FJ, Iqbal R, Ghani F, Siddiqui I, Khan AH. Bone health status of premenopausal healthy adult females in Pakistani females. Arch Osteoporos. 2012;7(1):93-99. doi: 10.1007/s11657-012-0085-0
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111(4):524-527. doi: 10.1016/j.jada.2011.01.004
- Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21(2):353-361. doi: 10.1681/ASN.2009040451
- Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25(2):141-148. doi: 10.1677/jme.0.0250141
- Tse AK, Zhu GY, Wan CK, Shen XL, Yu ZL, Fong WF. 1α, 25-Dihydroxyvitamin D3 inhibits transcriptional potential of nuclear factor kappa B in breast cancer cells. Mol Immunol. 2010;47(9):1728-1738. doi: 10.1016/j.molimm.2010.03.004
- Segersten U, Correa P, Hewison M, Hellman P, Dralle H, Carling T, et al. 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab. 2002;87(6):2967-2972. doi: 10.1210/jcem.87.6.8604
- Latacz M, Snarska J, Kostyra E, Fiedorowicz E, Savelkoul HF, Grzybowski R, et al. Single nucleotide polymorphisms in 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1) gene: the risk of malignant tumors and other chronic diseases. J Nutrients. 2020;12(3):1-22. doi: 10.3390/nu12030801
- Ferrer-Mayorga G, Larriba MJ, Crespo P, Munoz A. Mechanisms of action of vitamin D in colon cancer. J Steroid Biochem Mol Biol. 2019;185:1-6. doi: 10.1016/j.jsbmb.2018.07.002
- Silver J, Naveh-Many T. Vitamin D and the parathyroids. Vitamin D: Elsevier; 2018. p. 461-475. doi: 10.1016/B978-0-12-809965-0.00027-6
- Nishikawa M, Yasuda K, Takamatsu M, Abe K, Nakagawa K, Tsugawa N, et al. Generation of 1,25-dihydroxyvitamin D3 in Cyp27b1 knockout mice by treatment with 25-hydroxyvitamin D3 rescued their rachitic phenotypes. J Steroid Biochem Mol Biol. 2019;185:71-79. doi: 10.1016/j.jsbmb.2018.07.012
- Fabbri A, Infante M, Ricordi C. Editorial-Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. J Eur Rev Med Pharmacol Sci. 2020;24(7):4048-4052.
- Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol. 2019;30(5):733-743. doi: 10.1093/annonc/mdz059
- Jusu S, Presley J, Jean-Claude B, Stochaj U, Kremer R. Abstract LB-024: Inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1): evidence for impaired vitamin D signaling in an MMTV-PYMT mouse model of breast cancer. Cancer Res. 2019;79(13 Supplement):LB-024. doi: 10.1158/1538-7445.AM2019-LB-024
- Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11(9):1133-1146. doi: 10.2174/138945010792006799
- Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013;33(1):46-51. doi: 10.14694/EdBook_AM.2013.33.46
- Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: asystematic review of immune cell studies. PLoS One. 2015;10(11):1-12. doi: 10.1371/journal.pone.0141770
- Calton EK, Keane KN, Raizel R, Rowlands J, Soares MJ, Newsholme P. Winter to summer change in vitamin D status reduces systemic inflammation and bioenergetic activity of human peripheral blood mononuclear cells. Redox Biol. 2017;12:814-820. doi: 10.1016/j.redox.2017.04.009
- Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA. Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin D levels in blood: a novel approach to treat 25-hydroxyvitamin D deficiency. Antioxid Redox Signal. 2018;29(17):1792-1807. doi: 10.1089/ars.2017.7462
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319-329. doi: 10.1016/j.chembiol.2013.12.016
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
