By Kanwal Iqbal, Mervyn Hosein, Saima Butt, Afifa Razi
AFFLIATIONS:
Ziauddin College of Dentistry, Ziauddin University, Karachi, Pakistan.
Background: Oral submucous fibrosis (OSMF), a chronic debilitating condition distinguished by juxtaepithelial fibrosis or extensive fibrosis of submucosa and reduced vascularity, results in compromised blood supply causing tissue hypoxia. This brings about the transcription of a set of genes associated with angiogenesis, breakdown of iron/glucose, cell division and cell stability. Hypoxia Inducible Factor-1α (HIF-1α) is the main biomarker intervening in this reaction. The aim of research was to measure the salivary levels of HIF-1α in OSMF patients and healthy controls.
Methods: This study included 60 participants (30 Oral submucous fibrosis cases and 30 healthy controls). The consecutive sampling technique was used for the recruitment of the study participants. The enzyme-linked immunosorbent assay (ELISA) was used to evaluate HIF-1α levels in saliva. In order to determine data normality, the Kolmogorov-Smirnov test was used. Independent t test was applied to compare salivary levels of HIF-1α between cases and controls and p-value of less than 0.05 was considered statistically significant.
Results: The mean salivary HIF-1α value for the oral submucous fibrosis group was 20.18±7.78, compared to healthy controls 13.62±8.86. A statistically significant difference was seen between levels of HIF-1α in the OSMF and control group (p=0.003). The mean mouth opening for cases was 22.94±9.51 mm and for controls 42.00±5.19 mm. There was no correlation among salivary levels of HIF-1α in both the case and control groups (-0.059, -0.030) respectively.
Conclusion: Higher levels of HIF-1α were seen in the OSMF group in comparison to healthy individuals suggesting that HIF-1α may play a role in malignant transformation of OSMF.
Keywords: Oral Submucous Fibrosis; Saliva; Hypoxia Inducible Factor-1α.
Oral submucous fibrosis (OSMF) is a premalignant condition that has been portrayed as “an insidious, chronic disease that affects any part of the oral cavity and sometimes the pharynx and oesophagus. It is always associated with a juxtaepithelial inflammatory reaction followed by fibroelastic change of the lamina propria and epithelial atrophy leading to mucosal stiffness and functional morbidity” 1. Over five million people worldwide suffer from OSMF according to the World Health Organization (WHO) 2.The rate of malignant transformation of OSMF is about 7-30%, with a prevalence of about 0.03% to 6.42% 3,4. Epidemiological and in vitro experimental studies have established areca nut (in various preparations) as the principal etiological agent causing OSMF5. Due to the widespread availability of areca nut and smokeless chewable tobacco in Pakistan, it is consumed in large quantities contributing to the high frequency of OSMF in Pakistan6. According to a study carried out in a Pakistani village, 99 percent of OSMF patients had previously used areca nut7. A study conducted in southern Pakistan on schoolchildren eating areca nut reported the prevalence of OSMF ranged from 50% to 79.6%8.
Hypoxia Inducible Factor-1α (HIF-1α) is a transcriptional activator of genes that modulate oxygen homeostasis and metabolic activation. It is a key regulator for recognizing and responding oxygen levels in cells9. HIF-1α promotes angiogenesis in tumors by the generation of cellular vesicles, which facilitates intercellular contact at a distance. In hypoxic conditions, it is stabilized before translocating into the nucleus to interact with hypoxia responsive components. HIF-1α regulates the expression of genes that help cells to adapt and survive hypoxia10. In the early stages of OSMF, increased expression of HIF-1α induces transcription of downstream cytokines like TGF-β, PAI-1 and PDGF that increases proliferation of fibroblast and collagen synthesis while inhibiting collagen degradation, resulting in fibrosis11. Literature also showed an association between hypoxia and fibrosis in renal and lung fibroblasts12,13. IncreasedHIF-1α expression has been found in OSMF with risk of progression towards malignancy14. This could be attributed to fibrosis of connective tissue in OSMF and blood vessels constriction resulting in a reduced blood supply to the local tissue environment. Hence, it is considered as an important factor in the progression to malignant transformation15. In India and Sri Lanka, the association of HIF-1α with OSMF was investigated using immunohistochemistry and RT-PCR in tissue samples of OSMF, but no such study has been conducted in Pakistan. Furthermore, to the best of the author’s knowledge, no study has assessed the salivary levels of HIF-1α in OSMF. The purpose of this case control study was to compare the expression of salivary HIF-1α levels in OSMF patients and healthy controls.
This was a case control study and the consecutive sampling technique was used. Using OpenEpi software, the sample size was estimated. The study involved a total of 60 individuals, divided into 2 groups: 30 clinically diagnosed cases of oral submucous fibrosis and 30 clinically healthy individuals. Both study groups included males and females who had previously used areca nut and smokeless chewable tobacco. The exclusion criteria of this study are OSMF patients who had previously been treated with steroids or who had any chronic oral and systemic diseases. Ethical approval was taken from the Ethics Review Committee (ERC) of Ziauddin University, Karachi (Reference code: 1840120KIOM).
The protocol was described to all the participants who were asked to read the consent form and signed it before the start of the study. All participants were instructed that their participation was voluntary and they had the withdrawal right any time with no repercussions. One trained examiner distributed a complete and structured questionnaire to all the participants. Information about demographics, habits (e.g., use of areca nut, betel quid or gutka), duration and frequency of habit, clinical examination were included in the questionnaire for both the study groups.
All patients of OSMF were diagnosed clinically on the basis of: (a) inter- incisal mouth opening; (b) unilateral or bilateral fibrous bands in buccal mucosa; (c) buccal and palatal mucosal appearance; (d) a change in the appearance or movement of the tongue and; (e) and presence of soft palatal fibrosis. All saliva samples were collected from Department of Oral Medicine, and analyzed at Multidisciplinary Research Laboratory (MDRL), Ziauddin University, Clifton, Karachi. Unstimulated whole saliva (UWS) by passive drooling method was obtained from both the study groups and was done early in the morning. Salivary HIF-1α levels were determined by enzyme-linked immunosorbent assay technique (ELISA) kit (SEA798Hu, USCN, USA).
Statistical Package for Social Services (SPSS) version 22 was used for data analysis. For descriptive statistics percentage and frequency was reported for categorical variables. For continuous data, mean and standard deviation was calculated. Kolmogorov-Smirnov test was used to assess data normality. In inferential statistics, Independent t and Mann Whitney tests were used to compare salivary levels of HIF-1α and other characteristics between cases and controls respectively. Spearman correlation test was applied to report correlation between salivary HIF-1α levels and other variables like frequency, duration of tobacco product use and mouth opening. p-value < 0.05 was considered statistically significant.
The current research included 60 participants, 30 cases and 30 controls. The mean overall age of study subjects was 33.21±11.11. However, mean age for OSF cases was 35.56±12.55 and 30.86±9.06 for controls. Sixty-seven percent (n=20) of the cases were males and 33% (n=10) of cases were females. Similarly, 73% (n=22) of controls were males and 27% (n=8) were females (Table 1).
Table 1: Characteristics of the cases and controls.
| Characteristics | Cases
Mean ± SD |
Controls Mean ± SD | p-Value | |
| Gender n (%) | Male | 20 (67) | 22 (73.33) | 0.573 |
| Female | 10(33) | 8 (26.66) | ||
| Age | 35.56± 12.55 | 30.86± 9.06 | 0.242 | |
| Frequency of tobacco use | 17.06 ± 24.32 | 5.63 ± 5.28 | 0.001 | |
| Duration of tobacco use | 13.15 ±6.69 | 8.16 ± 6.63 | 0.005 | |
| Mouth opening | 22.94±9.51 | 42.00 ±5.19 | 0.001 | |
| Mean salivary HIF-1α levels | 20.18 ±7.78 | 13.62 ±8.86 | 0.003 | |
| Clinical characteristics of cases (OSMF) n (%) | ||||
| Buccal fibrous bands | Bilateral | 22(73) | ||
| Unilateral | 5(16.6) | |||
| No fibrosis | 3(10) | |||
| Tongue restriction | 12(40) | |||
| Without tongue restriction | 18(60) | |||
| Burning sensation | 11(36.6) | |||
| Without burning sensation | 19(63.3) | |||
Out of thirty OSF cases, 10 participants were exclusive betel nut chewers, 1 exclusive gutka user and 19 participants were using different tobacco products in combination. Also out of thirty healthy controls, 8 were exclusive betel nut chewers, 1 pan user, 3 smokers, 2 naswar users, 10 participants with all above habits and 6 controls had no tobacco eating habits. Frequency of use per day and duration of use in years are mentioned in Table 1. From the 30 cases, 5 patients had unilateral buccal fibrous bands, 22 patients had bilateral buccal fibrous bands (Figure 1) and 3 patients had no fibrous bands. Twelve cases had tongue restriction and fibrous soft palate whereas 18 cases showed absence of this finding. Eleven cases complained of a burning sensation, and 19 cases did not complain of a burning sensation.
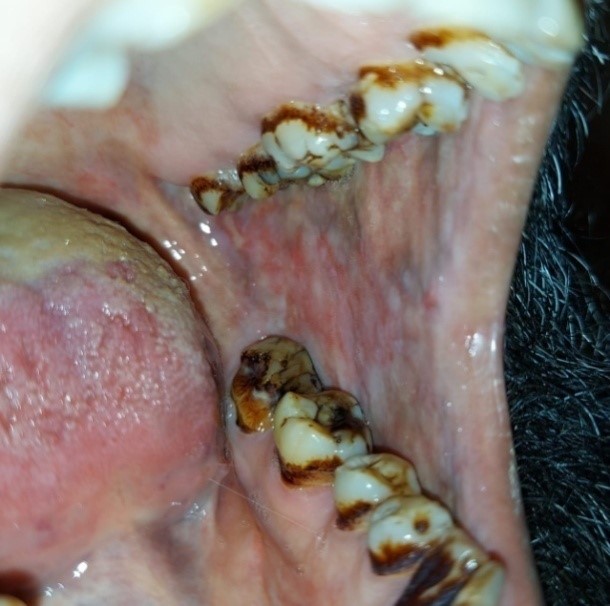
Figure 1: Clinical changes affecting the buccal mucosa showing significant blanching or marble-like appearance thus, noted severe fibrosis of the buccal mucosa.
The mean salivary HIF-1α levels were 20.18± 7.78 for OSMF group whereas mean salivary HIF-1α for healthy controls were 13.62 ± 8.86 (Figure 2). Independent t test showed statistically significant result (p=0.003) indicating significant difference between salivary levels of cases and controls (Table 1). The mean mouth opening for case was recorded as 22.94±9.51 mm and for controls 42.00±5.19 mm showed significant difference (p=0.001). Similarly frequency and duration of tobacco use showed significant difference among cases and controls (p=0.001, p=0.005), respectively (Table 1).
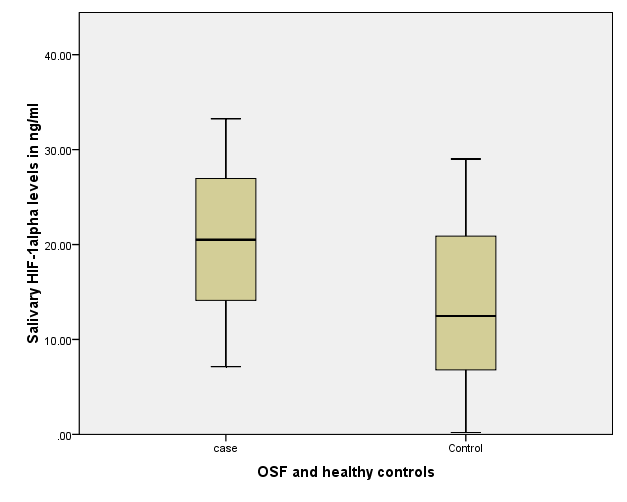
Figure 2: Box and Whisker plot representing mean concentrations of salivary HIF 1- alpha among OSMF cases and healthy control thus, noted higher levels in cases and low levels in controls of salivary HIF-1α.
Spearman correlation test was used to explore for any correlations among salivary HIF-1α levels and explanatory variables assessed in OSMF cases. Frequency of tobacco use i.e. number of packets per day, duration of use and mouth opening in millimeters showed no significant correlation with salivary levels of HIF-1α in case and control groups (Table 2).
Table 2: Correlation among Salivary HIF-1α levels, tobacco use and mouth opening.
| Variables | Cases (n=30) | Controls (n=30) | ||
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| Frequency of tobacco use
(no of packets per day) |
0.098 | 0.607 | 0.131 | 0.492 |
| Duration of tobacco use
(in years) |
-0.150 | 0.430 | 0.285 | 0.126 |
| Mouth Opening
(in mm) |
-0.059 | 0.757 | -0.030 | 0.876 |
*p-value < 0.05 was considered significant; Spearman correlation test was applied.
We hypothesized in this study that salivary HIF-1α levels in OSMF are higher than in healthy controls. The results of this study revealed a statistically significant difference in HIF-1α expression between cases and controls. Moreover, we also assessed correlation between salivary HIF-1α levels and clinical parameters but no significant correlation was found between them. However, there was a non-significant negative association found between salivary HIF-1α levels and mouth opening which means that if mouth opening is decreased then levels increased.
Due to connective tissue fibrosis in OSMF, there is blood vessels constriction resulting in a reduced blood supply to the local tissue environment. This hypoxia induces the activation of the transcription factor HIF-1α. Numerous studies reported overexpression of HIF-1α in OSMF as compared to controls but all of the studies were carried out on tissue samples of OSMF. Hande et al. evaluated the expression of HIF-1α in OSMF using immunohistochemistry16. However the comparison of our study with study by Hande and coworkers had certain limitations like the difference in sample used (saliva vs. tissue) and analytical techniques employed in the studies (ELISA vs. immunohistochemistry). Pereira, Dalmia, Chaudhry and Tilakaratne also evaluated HIF-1α expression in OSMF and controls and showed statistically significant results17-20. Again, these researches involved tissue biopsy, immunohistochemistry and polymerase chain reaction (PCR) for biomarker identification. To the best of our knowledge, the study is the first carried out on the salivary expression of HIF-1α in OSMF and healthy individuals. According to the findings of this study, salivary HIF-1α levels were increased in OSMF patients as compared to healthy individuals. As a result, the present study data suggested that HIF-1α could have a role in the malignant transformation of OSMF.
In this study, out of 30 cases 20 were males (67%) and 10 (33%) were females which clearly showed a male predominance. Similar observations have been reported in other studies21,22. This is due to easy accessibility of areca nut based products, use of areca nut and related products by co-workers/closely associated people at work place or people in the immediate surroundings. Moreover freedom from financial and social/cultural constraints in the use of these products among males also make them more prone to their use23. However recent studies have shown changing trends with an increase in incidence of use of areca base products among females as well24. This could be due to the social tolerability in use of areca nut based products by females, also due to the lack of knowledge and false beliefs of areca nut and related products being harmless and favorable products.
In the current study, the mean age of cases was calculated to be 35.56±12.55. Similar age group was reported in other studies as well25,26. OSMF is a condition that prevails in the 2nd and 3rd decades of life as results from several studies have shown that use of tobacco is more prevalent among younger individuals. According to epidemiological data, areca nut consumption in younger individuals is mostly associated with low/middle socioeconomic status and low cost and eases of access to these products27. Chewing areca nut, betel quid and gutka, raises the risk of developing OSMF. Areca nut contains a variety of biologically active alkaloids and flavonoids that stimulate fibroblast cells to promote collagen production while simultaneously reducing collagen degradation due to increased collagen structural stability and decreased collagenase activity28. Majority of the participants in our study had the combine habits of betel nut and gutka while some participants were exclusively betel nut chewers. The above findings are in accordance with other studies as well29,30.
This study, like many others, has some limitations that must be considered. We cannot generalize the findings because samples were solely collected from a single institute. It should be mentioned that the sensitivity/specificity of HIF-1α in saliva was not calculated in the present study. We used clinical examination only to diagnose OSMF in the present study. Pathologists can get diagnosis that is more accurate and staging of OSMF using invasive procedures like “punch biopsy”. These methods, however, have a major impact on the compliance of patient. OSMF is classified based on many clinical parameters and histopathological manifestations, which have been thoroughly established. As a result, determining the severity of the disease and its malignant transformation in relation to the levels of HIF-1α in saliva might be difficult. More research is needed to compare salivary HIF-1α levels in different stages of OSMF
In this study, higher level of HIF-1α was seen in oral submucous fibrosis (OSMF) group in comparison to the healthy individuals suggesting that HIF-1α may play a role in malignant transformation of OSMF.
The authors would like to thank Mr. Moazzam Ali Shahid for his cooperation and valuable guidance during the laboratory procedure.
The authors declare no conflict of interest.
Ethical approval was taken from the Ethics Review Committee (ERC) of Ziauddin University, Karachi with the Reference code (1840120KIOM).
KI did the conceptualization of study, literature search, data collection and written the article. MH, SB and AR did the proof reading and overall evaluation.
- More CB, Rao NR. Proposed clinical definition for oral submucous fibrosis. J Oral Biol Craniofac Res. 2019;9(4):311-314.
- Shih YH, Wang TH, Shieh TM, Tseng YH. Oral submucous fibrosis: A review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci. 2019;20(12):1-22.
- Das M, Manjunath C, Srivastava A, Malavika J, Ameena M. Epidemiology of oral submucousfibrosis: A review. Int J Oral Health Med Res. 2017;3(6):126-129.
- Peng Q, Li H, Chen J, Wang Y, Tang Z. Oral submucous fibrosis in Asian countries. J Oral Pathol 2020;49(4):294-304.
- Kondaiah P, Pant I, Khan I. Molecular pathways regulated by areca nut in the etiopathogenesis of oral submucous fibrosis. Periodontology. 2000. 2019;80(1):213-224.
- Raffat MA, Hadi NI, Hosein M, Zubairi AM, Ikram S, Akram Z. Differential expression of salivary S100A7 in oral submucous fibrosis. Saudi Dent J. 2019;31(1):39-44.
- Memon MA, Shaikh MS, Jaffery MH. Oral submucosal fibrosis in rural Sindh. J Liaq Univ Med Health Sci. 2015;14:44-47.
- Raffat MA, Hadi NI, Alghamdi O, Al-Aali KA, Al Deeb M, Abduljabbar T, et al. Expression of salivary S100A7 levels in stage I oral submucous fibrosis: A clinical and laboratory study. Asian Pac J Cancer Prev. 2020;21(4):1115-1119.
- Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27(2):281-298.
- Albanese A, Daly LA, Mennerich D, Kietzmann T, Sée V. The role of hypoxia-inducible factor post-translational modifications in regulating its localisation, stability, and activity. Int J Mol Sci. 2021;22(1):1-17.
- Shukla A, Singh A, Srivastava R. Oral submucous fibrosis: An update on etiology and pathogenesis-a review. Rama Univ J Dent Sci. 2015;2(1):24-33.
- Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, et al. Signalling pathways involved in hypoxia‐induced renal fibrosis. J Cell Mol Med. 2017;21(7):1248-1259.
- Senavirathna LK, Huang C, Yang X, Munteanu MC, Sathiaseelan R, Xu D, et al. Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Sci Rep. 2018;8(1):1-16.
- Chatterjee R, Ghosh B, Mandal M, Nawn D, Banerjee S, Pal M, et al. Pathophysiological relationship between hypoxia associated oxidative stress, epithelial-mesenchymal transition, stemness acquisition and alteration of Shh/Gli-1 axis during oral sub-mucous fibrosis and oral squamous cell carcinoma. Eur J Cell Biol. 2021;100(1):1-11.
- Ekanayaka R, Tilakaratne W. Oral submucous fibrosis: review on mechanisms of pathogenesis and malignant transformation. J Carcinog Mutagen. 2013:1-11.
- Hande AH, Chaudhary MS, Gadbail AR, Zade PR, Gawande MN, Patil SK. Role of hypoxia in malignant transformation of oral submucous fibrosis. J Datta Meghe Inst Med Sci Uni. 2018;13(1):38-43.
- Pereira T, Surve R, Shetty S, Gotmare S. Qualitative expression of hypoxia-inducible factor-1α in malignant transformation of oral submucous fibrosis: An immunohistochemical study. J Oral Pathol Med. 2020;24(1):106-112.
- Dalmia A, Hazarey V, Talkal R, Ganvir S, Purohit HJ, Gupta S, et al. Role of Hypoxia Inducible Factor-1α messenger RNA expression in malignant transformation of oral submucous fibrosis: A RT-PCR study. Transl Res Oral Oncol. 2016;1:1-5.
- Chaudhary M, Bajaj S, Bohra S, Swastika N, Hande A. The domino effect: Role of hypoxia in malignant transformation of oral submucous fibrosis. J Oral Maxillofac Pathol. 2015; 19(2):122-127.
- Tilakaratne W, Iqbal Z, Teh M, Ariyawardana A, Pitiyage G, Cruchley A, et al. Upregulation of HIF‐1α in malignant transformation of oral submucous fibrosis. J Oral Pathol 2008;37(6):372-377.
- Panda S, Panda BK, Pattnaik B, Naik C, Dany SS, Avijeeta A. Prevalence of oral submucous fibrosis in a tertiary care hospital of Odisha – A cross-sectional study. J Evid Based Med Healthc. 2020;7(49):1-4.
- Yang SF, Wang YH, Su NY, Yu HC, Wei CY, Yu CH, et al. Changes in prevalence of precancerous oral submucous fibrosis from 1996 to 2013 in Taiwan: A nationwide population-based retrospective study. J Formos Med Assoc. 2018;117(2):147-152.
- Srivastava R, Jyoti B, Pradhan D, Siddiqui Z. Prevalence of oral submucous fibrosis in patients visiting dental OPD of a dental college in Kanpur: A demographic study. J Family Med Prim Care. 2019; 8(8): 2612-2617.
- Mohiuddin S, Fatima N, Hosein S, Hosein M. High risk of malignant transformation of oral submucous fibrosis in Pakistani females: A potential national disaster. J Pak Med Assoc. 2016;66(11):1362-1366.
- Prathima V, Koneru M, Sunil V, Jois H, Reddy M. An 8-year retrospective analysis of oral submucous fibrosis in patients visiting dental College, Secunderabad. J Indian Assoc Public Health Dent. 2021;19(1):61-64.
- Patil DJ, Joshi M. Evaluation of hematological profile in oral submucous fibrosis: A cross-sectional study. J Oral Maxillofac Pathol. 2020;24(3):1-9.
- Acharya S, Rahman S, Hallikeri K. A retrospective study of clinicopathological features of oral squamous cell carcinoma with and without oral submucous fibrosis. J Oral Maxillofac Pathol. 2019;23(1):1-8.
- Arakeri G, Rai KK, Hunasgi S, Merkx M, Gao S, Brennan PA. Oral submucous fibrosis: An update on current theories of pathogenesis. J Oral Pathol 2017;46(6):406-412.
- Desai KM, Kale AD, Angadi PV, Datar UV. Clinicopathological evaluation of oral submucous fibrosis-a retrospective, single institute study. Annals of Dental Specialty Vol. 2021;9(1):27-33.
- Mahajan SS, Ramani P. Association of habits with clinical symptoms in oral submucous fibrosis patients-a retrospective study. J Contemp Issues Busin Govern. 2020;26(2):176-183.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
