By Syed Mehmood Hasan1, Faiza Zeeshan1, Asma Shabbir1, Amber Ilyas2, Syed Muhammad Hasan3, Masood Husain4
AFFLIATIONS:
- Department of Pathology, Sindh Medical College, Jinnah Sindh Medical University, Karachi, Pakistan.
- Department of Anatomy Sindh Medical College Jinnah Sindh Medical University, Karachi, Pakistan.
- Department of Medicine, National Institute of Diabetes and Endocrinology, Dow University of Health Sciences, Karachi, Pakistan.
- Tahir Laboratory, Karachi, Pakistan.
Background: The emergence of antibiotic resistance among pathogens causing urinary tract infections (UTI) has made treatment options limited. The use of fosfomycin along with other drug combination can significantly address this problem. Our study aimed to identify the rate of resistance among uropathogens and their susceptibility patterns to fosfomycin along with other antibacterial agents.
Methods: The retrospective study was conducted at Jinnah Sindh Medical University in collaboration with Dr. Tahir Laboratory, Karachi. A total of 146 urine samples were included which were processed for antibacterial susceptibility testing by Kirby-Bauer disk diffusion method and rate of resistance for antibacterial agents especially fosfomycin were recorded. The statistical analysis was performed by using Chi squared tests and p>0.05 was considered statistically significant.
Results: The study reported lowest rate of resistance for fosfomycin among Escherichia coli 3(5.3%), Klebsiella pneumoniae 7(14%) and Pseudomonas aeruginosa 9(22.5%) in comparison with ampicillin, which showed resistance in 43(76.8%), 41(82%) and 39(97.5%) cases of E. coli, K. pneumoniae and P. aeruginosa respectively. The subgroup carbapenem resistant Enterobacteriaceae (CRE) and extended spectrum β-lactamases (ESBLs) producers were seen noticeably high in P. aeruginosa. Overall, the female to male ratio was 1.4:1 (87/59), showing female preponderance (p=0.02). A majority of patients belonged to adult age group (61.6%) followed by senior adults (23.2%, p=0.05).
Conclusion: High levels of resistance to commonly used antibiotics were observed. The increasing rate of resistance among Enterobacteriaceae to cephalosporin and ampicillin is an alarming situation. In this context, fosfomycin is an interesting alternative option in treatment of complicated and uncomplicated urinary tract infections.
Keywords: Antibiotic Resistance; Enterobacteriaceae; Extended Spectrum Beta Lactamase; Fosfomycin; Urinary Tract Infection.
The antibacterial resistance has become an exasperating yet challenging issue globally. The irrational use of antibiotics in the field of human and veterinary medicine, agriculture and farming has brought the epoch of antibacterial drugs, nearly to an edge. The antibiotic resistance has shown an increment of 9% from 2014 to 2017 in United Kindom1. In United State of America, the multidrug resistant uropathogens have been reported in 7% to 13% cases in last decade2. The condition in Asian countries is even more serious, the prevalence of resistant uropathogens is reported as 38.3% by SMART (Study for Monitoring Antimicrobial Resistance Trends) program2. The bacterial resistance has become an alarming sign in urological practices in general, and urinary tract associated infections in particular. The extended spectrum beta lactamase (ESBL) producing Enterobacteriaceae in context to uropathogens have become a complex issue when treatment modalities are concerned.
The ESBL bacteria exhibit resistance towards third generation cephalosporins, penicillins and monobactams. The ESBL E. coli, K. pneumonia, Pseudomonas species, Proteus species and other uropathogens have shown decreased activity against cephalosporins, quinolones, and aminoglycosides and even with cephamycins3. Trimethoprim-sulfamethoxazole has also become ineffective in ESBL pathogens and treatment of urinary tract infections by a single potent drug is becoming difficult day by day. The scenario is more complicated with hospital associated urinary tract infections where resistant strains of Pseudomonas and Acinetobacter baumannii are found to be involved. Therefore, the advent and practice of newer antibacterial drugs is current era’s most pivotal requirement.
Fosfomycin- promethazine is a synthetic, broad spectrum, bactericidal antibiotic that works by inhibiting pyruvyl-transferase during bacterial cell wall synthesis, and it decreases bacterial adherence to epithelial cells in the urinary tract. Fosfomycin is considered as first line of therapy for uncomplicated UTI4. Fosfomycin has shown efficacy in both in vitro and in vivo studies for treating UTIs caused by ESBL-producing Enterobacteriaceae and vancomycin resistant enterococci5. In addition, it is an alternative option for multidrug resistant (MDR) organisms, allergies where the intravenous antibiotics cannot be recommended6. The recent studies have reported its activity against methicillin resistant Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pneumoniae7.
In developing countries like Pakistan, where health care facilities are inadequate, the emergence of resistant bacterial strains is no lesser than havoc. Data from Pakistan regarding susceptibility patterns towards fosfomycin is limited and newer studies are required in order to get awareness of current scenario in Pakistan. Therefore, this study was designed to get insight of fosfomycin susceptibility patterns among local population.
This retrospective study was conducted at Jinnah Sindh Medical University after getting ethical approval from Institutional Review Board (JSMU/IRB/2019/274) and Dr. Tahir laboratory, Hamdard University and hospital. A total of 146 urine samples which showed positive bacterial isolates collected between the time periods of July 2019 to July 2020, were included in the study. The samples were collected and processed in Dr. Tahir Laboratory. The data regarding patient’s age, gender and registration number and isolated uropathogens belonging to Enterobacteriaceae were recorded. The cases with incomplete data were excluded from study.
The urine samples were collected in sterile screw capped containers and was properly labeled. The samples were inoculated on Blood, MacConkey’s and cysteine lactose electrolyte deficient (CLED) agar. The identification of bacterial isolates was made by appropriate biochemical tests and was categorized as extended spectrum beta lactamases (ESBLs) and carbapenem resistant Enterobacteriaceae (CRE) based on sensitivity guidelines provided by Clinical Laboratory Standard Institute (CLSI, United States of America, 2020). The organisms which showed resistance towards third generation cephalosporins (ceftazidime, ceftriaxone and cefixime), monobactams (aztreonam) and were inhibited by β Lactamase inhibitors (clavulanate, sulbactam and tazobactam) were termed as ESBLs8. On the other hand, the Enterobacteriaceae, which mediated resistance towards carbapenems (ertapenem, meropenem and imipenem), were termed as CREs9.
The antibacterial susceptibility testing (AST) was performed by Kirby-Bauer’s disc diffusion method on Muller-Hinton agar. The zone of inhibition around antibiotic discs was measured and organisms were considered sensitive, intermediate or resistance according to the cut off values provided by CLSI guidelines 2020. The antibiotic discs were purchased from Oxoid (United Kingdom). Following antibiotic discs were used in AST: amoxicillin- clavulanic acid (20/10µg), ampicillin (10µg), cefotaxime (30µg), ceftazidime (30µg), cefepime (30µg), cefuroxime (30µg), aztreonam (30µg), gentamicin (10µg), amikacin (30µg), ciprofloxacin (5µg), nalidixic acid (30µg), trimethoprim-sulfamethoxazole (30µg), nitrofurantoin (300µg) and fosfomycin (200µg).
The screening test for ESBL was performed by the method proposed by CLSI 10. E. coli ATCC 25922 was used as quality control strains. The bacterial isolates were supposed to be ESBL, when the zone of inhibition for ceftazidime was ≤22mm and for cefotaxime was ≤27mm respectively. The double disc synergy test was used to confirm ESBL producers. In this test, amoxicillin-clavulanic acid (20/10μg) was placed on Muller-Hinton agar plate, which was already streaked with testing organism. The discs for ceftazidime (30μg) and cefotaxime (30μg) were placed about 20mm apart from amoxicillin-clavulanic acid discs. Those phenotypes, which showed phenomenon of synergism, were confirmed as ESBL producers.
The CREs were detected by performing sensitivity testing of imipenem, ertapenem and meropenem on Muller-Hinton agar by Kirby Bauer disc diffusion method. The break point for imipenem was ≤19mm while for ertapenem and meropenem was taken ≤21mm (proposed by CLSI) 10. The data were recorded in Statistical Package for Social Sciences (SPSS, IBM USA, version 22). The descriptive statistics and chi square tests were used to analyze the data. The data was presented in the form of tables, graphs and figures.
The study included 146 bacterial isolates of E. coli, K. pneumoniae and P. aeruginosa belonging to Enterobacteriaceae. There were 56 (38.3%) E. coli strains, 50 (34.2%) K. pneumoniae isolates while in 40 (27.3%) cases, P. aeruginosa was recovered. Overall, the female to male ratio was 1.4:1 (87/59), showing female preponderance (p=0.02) as shown in Table 1. A majority of patients belonged to adult age group (61.6%) followed by senior adults (23.2%, p=0.05).The E. coli was also most commonly observed in patients falling in adult age group (73.2%), followed by geriatric group (14.3%). The female to male ratio for positivity towards E. coli was 1:0.75 (24/32). The K. pneumoniae was prevalent in adult age group (58%) and being least common in adolescents (2%). The female to male ratio for UTI caused by K. pneumoniae was found to be 1:0.6(30/20). The P. aeruginosa also showed the same trend for being commonly observed in adults (73.2%) and least prevalent among children (5.4%). The female to male ratio for K. pneumoniae was 1.6:1 (25/15).
Table 1: Demographic characteristics of the study population.
| Bacterial Isolates | Gender | Age group | Total | |||||
| Male (n) |
Female
(n) |
F:M Ratio | Children (0-10 years) |
Adolescents (11-19 years) |
Adults (20-59 years) |
Senior adults (60 and above) |
||
| Escherichia coli
|
24 | 32 | 1:0.75 | 3 | 4 | 41 | 8 | 56 |
| Klebsiella pneumoniae
|
20 | 30 | 1:0.6 | 5 | 1 | 29 | 15 | 50 |
| Pseudomonas aeruginosa | 15 | 25 | 1.6:1 | 2 | 7 | 20 | 11 | 40 |
| Total | 59 | 87 | 1.4:1 | 10 | 12 | 90 | 34 | 146 |
| p-Value | 0.02 | 0.05 | 0.05 | |||||
The rate of resistance for various antimicrobial agents among Enterobacteriaceae is shown in Table 2. The E. coli strains were highly resistant to penicillin group (76.8%) followed by cephalosporins (cefaclor 48.2%, ceftriaxone 41.1% and ceftazidime 37.5%). The E. coli isolates were found to be least resistant for fosfomycin (5.3%). About 10.8% samples of E. coli were CRE and 41.78% were ESBL producers.
Table 2: Resistance of uropathogens to various antibacterial agents.
| Antibiotic Class | Antibacterial* Agent | Escherichia coli
n (%) |
Klebsiella pneumoniae
n (%) |
Pseudomonas aeruginosa
n (%) |
| Fosfomycin | FOS | 3(5.3) | 7(14) | 9(22.5) |
| Β-lactamase inhibitors | AMC | 13(23.2) | 38(76) | 29(72.5) |
| Penicillins | AMP | 43(76.8) | 41(82) | 39(97.5) |
| Cephalosporins | CFM | 20(35.7) | 28(56) | 23(57.5) |
| CRO | 23(41.1) | 29(58) | 19(47.5) | |
| CEC | 27(48.2) | 22(44) | 22(55) | |
| CAZ | 21(37.5) | 21(42) | 25(62.5) | |
| CTX | 26(46.4) | 26(52) | 25(62.5) | |
| Folate pathway inhibitor | SXT | 20(35.7) | 20(40) | 27(67.5) |
| Monobactams | ATM | 15(26.8) | 24(48) | 21(52.5) |
| Quinolones | CIP | 15(26.8) | 23(46) | 16(40) |
| Aminoglycosides | AK | 4(7.2) | 9(18) | 13(32.5) |
| CN | 14(25) | 11(22) | 16(40) | |
| Nitrofurantoin | F | 11(19.6) | 13(26) | 11(27.5) |
| Carbapenem | MEM | 6(10.8) | 3(26) | 16(40) |
Abbreviations* FOS=fosfomycin, AMC=ampicillin-clavulanate, AMP=ampicillin, CFM=cefixime CRO=ceftriaxone, CEC=cefaclor CAZ=ceftazidime, CTX=cefotaxime TS=trimethoprim-sulfamethoxazole, ATM=aztreonam, CIP=ciprofloxacin, AK=amikacin, CN=gentamicin, F=nitrofurantoin, MEM=meropenem
The UTI was found to be more common among females, however relationship between gender and resistance for antimicrobial agents was not established (p>0.05). Similarly, there was no association found between age groups and drug resistance (p>0.05).
The K. pneumoniae showed highest rate of resistance for penicillins (76%). The K. pneumoniae isolates were found to be considerably resistant for quinolone group (46%). The rate of resistance was also high among cephems (ceftriaxone 58.2%, cefaclor 44%, and ceftazidime 42%). The fosfomycin came up as the most potent agent against K. pneumoniae, being 14% resistant in all cases. The frequency of ESBL producers was 50.44, while 26% isolates were CRE (Figure 1).
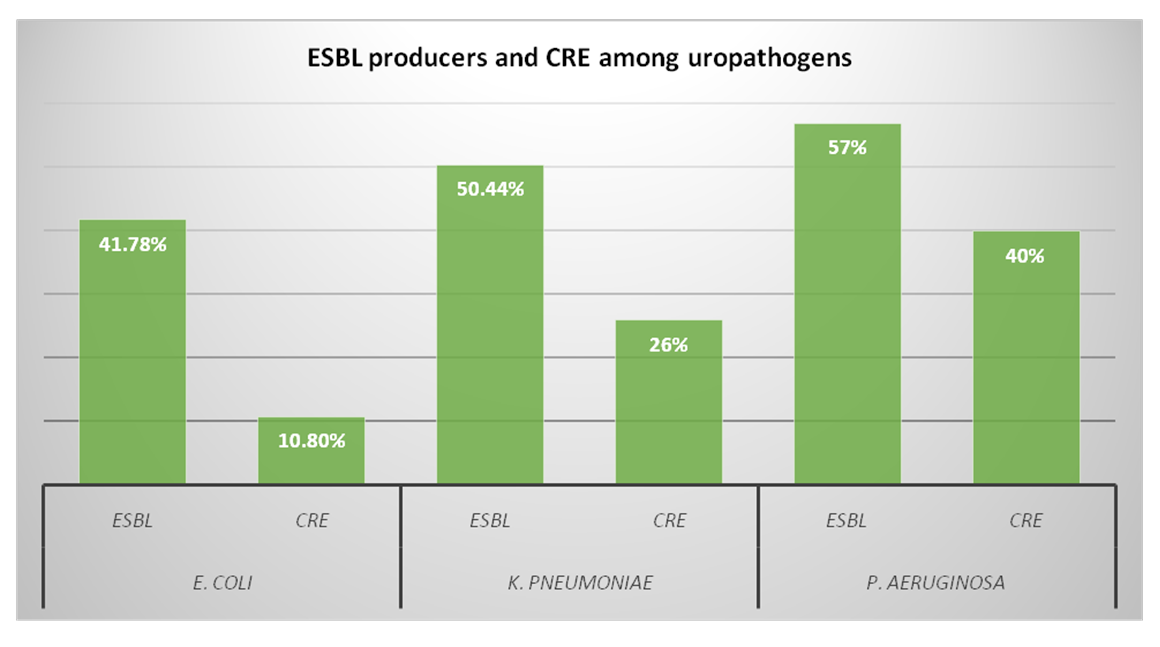
Figure 1: Distribution of extended spectrum beta lactamase (ESBL) producers and carbapenem resistant Enterobacteriaceae (CRE) among uropathogens.
The rate of resistance in P. aeruginosa for penicillin group was significantly high (97.5%) which was followed by folate pathway inhibitors (67.5%).The resistance for cephalosporins was also substantially high (ceftazidime 62.5%, cefaclor 55%, ceftriaxone 47.5%). The 57% P. aeruginosa isolates were ESBL producers while 40% fall in CRE group. The resistance towards fosfomycin was observed as 22.5% being highest among all three pathogens (Figure 2).
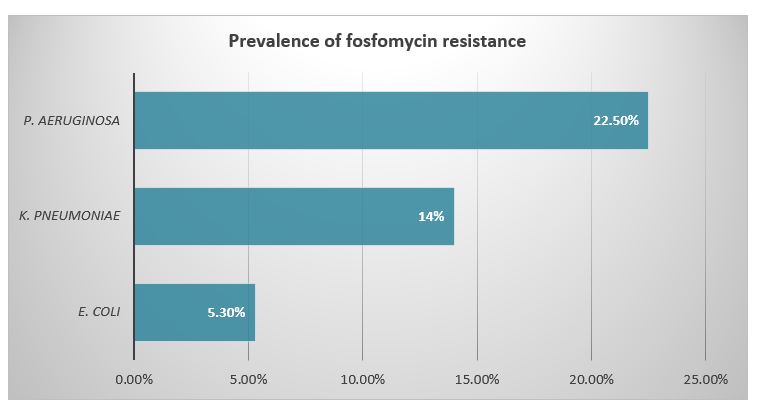
Figure 2: Prevalence of fosfomycin resistance among Enterobacteriaceae
The current study was conducted to evaluate the antibiotic susceptibility patterns of uropathogens belonging to Enterobacteriaceae for fosfomycin in particular. Urinary tract infection (UTI) is one of the commonest infectious diseases, affecting outpatients as well as hospitalized individuals. It is estimated that the lifetime incidence of uncomplicated UTI among adult and sexually active women is about 50-60% 11. This incidence increases by 20% with increasing age in females12. Our study also demonstrated the increased frequency of UTI in female population in comparison with males. Similar results have been reported by Mohammed et al. and Sewify et al 13,14. The females are more prone to contract UTI because of several factors including short urethra, sexual activity, compromised hygienic practices and low levels of estrogen after menopause15. According to our study, the age group, which affected most, was adult group i.e. between 20-59 years of age. Our results were in complete agreement with Tan and Chlebicki and Kabugo et al16,17. The reasons behind increased prevalence among adults are dependent upon sexual behaviors, use of contraceptive devices, pregnancy, urethral strictures and renal stones18.
Gram-negative Enterobacteriaceae, being positive in 75% to 95% cases, carries the major UTI burden18. The increasing rate of resistance among uropathogens has become an agonizing problem globally, which has not savaged even developed world. Pakistan is experiencing the similar serious threat of antimicrobial resistance. The present study showed the rising frequency of extended spectrum beta lactamases (ESBL) producers and carbapenem resistant Enterobacteriaceae (CRE). The most common isolated pathogens in our study were E. coli (38.3%) followed by K. pneumoniae (34.2%) and P. aeruginosa (27.3%). These results were in accordance with Yeganeh-Sefidan et al. and Qamar et al. who also documented the same results19,20. The penicillin group was found to be least active against aforementioned uropathogens in vitro susceptibility testing. The P. aeruginosa showed highest rate of resistance (97.5%) towards ampicillin, which was followed by E. coli (76.8%) and K. pneumoniae (76%). Yeganeh-Sefidan et al., Yekani et al. and Lake et al. also reported the highest resistance of uropathogens towards ampicillin19,21,22. The resistance to ciprofloxacin, trimethoprim-sulfamethoxazole and gentamicin was also noteworthy. The K. pneumoniae isolates showed highest rate of resistance (44%) to ciprofloxacin in comparison with P. aeruginosa and E. coli, which showed 40%, and 26.8% resistance respectively. Reports by Reis et al. reinforced the results in present study, indicating increasing resistance to ciprofloaxcin23. The results by Qiao et al. are however contradictory to our results who stipulated higher sensitivity rates of uropathogens to ciprofloxacin24. The resistance to ciprofloxacin is conferred by single gene mutation and colonization by ciprofloxacin resistant Enterobacteriaceae causes the treatment burden and eventually killing of ciprofloxacin susceptible starins25. The ciprofloxacin is one of the most commonly prescribed antibiotics for uncomplicated UTI in Pakistan; rising incidence of resistance is indeed an alarming sign.
The resistance to trimethoprim-sulfamethoxazole (co-trimoxazole) among uropathogens was also significant. The Pseudomonas were highly resistant (67.5%) to the co-trimoxazole, our results showed complete agreement with Yekani et al21. The aminoglycosides are also prescribed for UTI and cystitis and according to our results; highest rate of resistance to gentamicin was seen in P. aeruginosa followed by E. coli (25%) and Klebsiella (22%). Gajdács and Urbán also documented the increasing resistances for aminoglycosides26. On contrary, the amikacin was found to be highly sensitive drug for all uropathogens. The frequencies of resistance by E. coli, K. pneumoniae and P. aeruginosa were found to be 6.2%, 10.8% and 9.7% respectively.
The third generation cephalosporins are used worldwide to treat complicated and hospital associated infections. This leads to emergence of third generation resistant Enterobacteriaceae (3GCREB) particularly among ESBL producers27. Often the ESBL production is co related with resistance to trimethoprim-sulfamethoxazole, aminoglycosides and quinolones as the same plasmids encodes resistance genes, which are responsible for ESBL production19. In the present study, the prevalence of ESBLs in E. coli was as high as 38.4%, While in K. pneumoniae and P. aeruginosa; it was found to be 56% and 47.5% respectively. Our results are in line with Rizzo et al. who reported the resistance by E. coli to 3rd generation cephalosporins as 33.9% and 26.9% respectively27. According to current study, the frequency of ESBL production among K. pneumoniae was 56% and in P. aeruginosa was 47.5%, which clearly reflects the higher incidence of ESBL producers among Enterobacteriaceae. The carbapenem resistant Enterobacteriaceae (CREs) are related significantly with morbidity and mortality. The prevalence of CRE in the present study was found to be noticeable among uropathogens.
The present study clearly demonstrates the highest rate of sensitivity of all three isolated bacterial species to fosfomycin. According to our results, overall rate of resistance among all isolates to fosfomycin was low. The P. aeruginosa showed higher rates of resistance to fosfomycin as compare to E. coli and Klebsiella species. The reason for low level of resistance among E. coli to fosfomycin is usually that fosfomycin is not prescribed for uncomplicated UTI. These stipulated results were in accordance with Yeganeh-Sefidan et al. conversely Huang et al. presented higher rates of resistance among Enterobacteriaceae to fosfomycin 19,28. According to Cao et al., the rate of resistance among E. coli isolates was as low as 4.6%, which is comparable to the current study results29. The rate of resistance by K. pneumoniae in our study was found to be 6.7% so; similar results were documented by van den Bijllaardt et al30.
The present study highlighted the effectiveness of fosfomycin in urinary tract infections especially by E. coli, K. pneumoniae and P. aeruginosa. The findings suggest the prompt and regular vigilance of antimicrobial resistance among uropathogens so that emergence of resistance by bugs can be controlled.
We would like to acknowledge the staff of Department of Pathology of Jinnah Sindh Medical University for facilitating the study.
The authors declare no conflict of interest.
Institutional Review Board (IRB) of Jinnah Sindh Medical University approved the study with reference number: JSMU/IRB/2019/274.
Consents were taken from all the patients selected in study.
SMH contributed in data collection, manuscript writing and review, FZ conceived the idea, did manuscript writing and data analysis, AS did data entry, contributed in manuscript writing and data analysis, SMH contributed in data collection, data analysis and review, and MH helped in data collection and data entry.
- Boycott-Oven M. Number of infections resistant to antibiotics rises by 9% in one year. The Telegraph [Newspaper on the internet] 2019 Oct 31; Health; [about 2pgs]. Available from https://www.telegraph.co.uk/news/2019/10/31/number-infections-resistant-antibiotics-rises-9-one-year/#
- Zowawi HM, Harris PN, Roberts MJ, Tambyah PA, Schembri MA, Pezzani MD, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12(10):570-584.
- Kaye KS, Pogue JM. Infections caused by resistant gram‐negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35(10):949-962.
- Giancola SE, Mahoney MV, Hogan MD, Raux BR, McCoy C, Hirsch EB. Assessment of fosfomycin for complicated or multidrug-resistant urinary tract infections: patient characteristics and outcomes. Chemotherapy. 2017;62(2):100-104.
- Gardiner BJ, Stewardson AJ, Abbott IJ, Peleg AY. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr. 2019; 42(1): 14-19.
- Bassetti M, Graziano E, Berruti M, Giacobbe DR. The role of fosfomycin for multidrug-resistant gram-negative infections. Current opinion in infectious diseases. 2019;32(6):617-625.
- Silver LL. Fosfomycin: mechanism and resistance. Cold Cold Spring Harb Perspect Med. 2017;7(2):1-12.
- Chowdhury AH, Nandi S, Rahman M, Karim AA, Mamtaz SS, Ara NN, et al. Comparison between phenotypic confirmatory test & double disc synergy test in detection of extended spectrum b-lactamases producers among gram-negative bacilli. Chattagram Maa-O-Shishu Hosp Med Coll J. 2016;15(2):3-8.
- Humphries RM, Hindler JA, Epson E, Horwich-Scholefield S, Miller LG, Mendez J, et al. Carbapenem-resistant Enterobacteriaceae detection practices in California: what are we missing? Clin Infect Dis. 2018;66(7):1061-1067.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 2019, 29th CLSI Supplement M100;Wayne.
- Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:1-5.
- Chu CM, Lowder JL. Diagnosis and treatment of urinary tract infections across age groups. Am J Obstet Gynecol. 2018;219(1):40-51.
- Mohammed MA, Alnour TM, Shakurfo OM, Aburass MM. Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac J Trop Med. 2016;9(8):771-6.
- Sewify M, Nair S, Warsame S, Murad M, Alhubail A, Behbehani K, et al. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. Journal of diabetes research. 2016;2016:1-7.
- John AS, Mboto CI, Agbo B. A review on the prevalence and predisposing factors responsible for urinary tract infection among adults. Euro J Exp Bio. 2016;6(4):7-11.
- Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016; 57(9): 485-490.
- Kabugo D, Kizito S, Ashok DD, Kiwanuka AG, Nabimba R, Namunana S, et al. Factors associated with community-acquired urinary tract infections among adults attending assessment centre, Mulago Hospital Uganda. Afr Health Sci. 2016;16(4):1131-1142.
- Kline KA, Lewis AL. Gram‐positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Urinary Tract Infect Mol Pathogen Clin Manag. 2017:459-502.
- Yeganeh-Sefidan F, Ghotaslou R, Akhi MT, Sadeghi MR, Mohammadzadeh-Asl Y, Baghi HB. Fosfomycin, interesting alternative drug for treatment of urinary tract infections created by multiple drug resistant and extended spectrum β-lactamase producing strains. Iran J Microbiol. 2016; 8(2):125-131.
- Qamar S, Shaheen N, Shakoor S, Farooqi J, Jabeen K, Hasan R. Frequency of colistin and fosfomycin resistance in carbapenem-resistant Enterobacteriaceae from a tertiary care hospital in Karachi. Infect Drug Resist. 2017; 10: 231-236.
- Yekani M, Baghi HB, Sefidan FY, Azargun R, Memar MY, Ghotaslou R. The rates of quinolone, trimethoprim/sulfamethoxazole and aminoglycoside resistance among Enterobacteriaceae isolated from urinary tract infections in Azerbaijan, Iran. GMS Hyg Infect Control. 2018; 13: 1-6.
- Lake JG, Weiner LM, Milstone AM, Saiman L, Magill SS, See I. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011–2014. Infect Control Hosp Epidemiol. 2018; 39(1):1-11.
- Reis AC, Santos SR, Souza SC, Saldanha MG, Pitanga TN, Oliveira RR. Ciprofloxacin resistance pattern among bacteria isolated from patients with community-acquired urinary tract infection. Revista do Instituto de Medicina Tropical de São Paulo. 2016;58:1-6.
- Qiao LD, Chen S, Yang Y, Zhang K, Zheng B, Guo HF, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open. 2013;3(12):1-7.
- Stewardson AJ, Vervoort J, Adriaenssens N, Coenen S, Godycki-Cwirko M, Kowalczyk A, et al. Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect. 2018;24(9):972-979.
- Gajdács M, Urbán E. Comparative epidemiology and resistance trends of proteae in urinary tract infections of inpatients and outpatients: A 10-year retrospective study. Antibiotics. 2019;8(3):1-13.
- Rizzo K, Horwich-Scholefield S, Epson E. Carbapenem and cephalosporin resistance among Enterobacteriaceae in healthcare-associated infections, California, USA. Emerg Infect Dis. 2019; 25(7):1389-1393.
- Huang L, Hu YY, Zhang R. Prevalence of fosfomycin resistance and plasmid-mediated fosfomycin-modifying enzymes among carbapenem-resistant Enterobacteriaceae in Zhejiang, China. J Med Microbiol. 2017;66(9):1332-1334.
- Cao XL, Shen H, Xu YY, Xu XJ, Zhang ZF, Cheng L, et al. High prevalence of fosfomycin resistance gene fosA3 in bla CTX-M-harbouring Escherichia coli from urine in a Chinese tertiary hospital during 2010–2014. Epidemiol Infect. 2017;145(4):818-824.
- van den Bijllaardt W, Schijffelen MJ, Bosboom RW, Cohen Stuart J, Diederen B, Kampinga G, et al. Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J Antimicrob Chemother. 2018;73(9):2380-2387.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
