By Syed Jaffar Abbas Zaidi1, Shakeel Kazmi2
AFFLIATIONS:
- Department of Oral Biology, Dow Dental College, Dow University of Health Sciences, Karachi, Pakistan.
- Department of Oral Biology, School of Dentistry, Shaheed Zulfiqar Ali Bhutto Medical University, Karachi, Pakistan.
Minimally invasive dentistry (MID) is an evidence based approach which mostly focuses on the repair and regeneration of dental tissues by dental materials. MID encompasses all dental specialities from minimally invasive approach to oral surgeries and impactions, minimally invasive restorative procedures, socket preservation techniques to minimally invasive cavity preparations to minimally invasive approach to caries control. There is a need to investigate the dental biomaterials proposed for MID in terms of their ease of use, bioactivity, biocompatibility, microleakage and longevity. An electronic search was performed in databases included PubMed, Scopus, and SciELO etc. with keywords: “dental biomaterials” and “minimally invasive dentistry”. Studies revealed that two commercially available products Mineral Trioxide Aggregate and Biodentine are first-line dental biomaterials for minimally invasive dentistry. Biodentine, a novel biomimetic and bioactive by Septodont, fulfils the goals of minimally invasive dentistry due to its bioactivity and enhanced biomechanical properties. Material testing needed to market this material has been critically analysed in light of the current standards and guidelines with due emphasis given to the drawbacks of Biodentine and remedies proposed in the form of another bioactive material, which could revolutionize the world of minimally invasive dentistry as the ultimate replacement material for enamel, dentin, and cementum.
Keywords: Minimally Invasive Surgical Procedures; Biomimetic Materials; Dentistry.
An ideal minimally invasive dental biomaterial is such that will conserve sound tooth structure, reinforce and repair the remaining tooth and possibly regenerate the tooth structure lost1. Minimally Invasive Dentistry (MID) encompasses all the dental specialities2. From minimally invasive surgeries to minimally invasive socket preservation techniques, minimally invasive impactions to minimally invasive cavity preparation techniques and minimally invasive restorations such as veneers; MID is here to stay, as it is patient and tooth structure friendly3-5. MID focuses on tissue preservation, thereby preventing tissue loss and avert the occurrence and spread of the disease. Obstructive sleep apnoea is now managed through minimally invasive procedures and restorative materials6. Adhesive materials, cariostatic materials and bioactive materials have led to increased interest in the field of MID7,8. Dentists are constantly replacing old restorations and it constitutes more than half of their practice9. The advancement in the diagnosis of caries and caries risk assessment measures has decreased the need for early restoration10. The original Black’s principles of cavity preparation of Extension for prevention” has been renamed by MID as “Prevention from Extension”9. From tunnel preparations and resin infiltration of incipient carious lesions, to repair instead of replacement of defective restorations, the concept of MID has become successful through the introduction of bioactive dental biomaterials11.
An electronic search was performed in five databases: PubMed, Scopus, and Directory of open access journals (DOAJ), Web of Science, and SciELO. The following keywords: “dental biomaterials” and “minimally invasive dentistry” were used for the searches. Search strings were built based on Medical Subject Headings (MeSH), Descriptors in Health Sciences (DeCS) and other means of Boolean operators (e.g., “AND”, “OR”) depending on the searched databases. For regional and non-indexed journals, Google Scholar was used for the searches. The literature search resulted in 186 papers. Out of these only 80 papers were included in which bioactive dental materials were investigated for use in minimally invasive dentistry. For local journals pakmedinet.com, which is the local search engine in Pakistan was used. The search for keywords resulted in four studies. All of these local studies were either review articles of KAP studies and no original study was found.
Quest for such an ideal minimally invasive material is still going on. There is a huge debate amongst clinicians regarding the claim of superiority of one restorative biomaterial over the other such as composites and glass ionomers in line with MI philosophy in the management of caries12. Calcium silicate-based materials are dentin replacement materials that have been recently marketed with the claim of bioactivity13. Bioactive materials result in better adaptability of the materials to the dental hard tissues and result in longevity of restoration. Although there are various dental biomaterials commercially marketed for minimally invasive dentistry, their claims, and merits in terms of bioactivity and drawbacks need to be investigated in light of the testing requirements for their efficacy and longevity. The objective of this clinical review is to identify bioactive dental biomaterials and their clinical indications in MID and to investigate the material testing requirements for these dental materials for their merits and demerits.
This review focuses on an overview of ideal properties of dental biomaterials for minimally invasive dentistry, clinical review of commercially available dental biomaterials for MID, their material testing requirements and their merits and demerits. Some evidence-based recommendations of modifications to current biomaterials to maximize their bioactivity will be presented.
Properties of Ideal Dental Materials for Minimally Invasive Dentistry
The ideal properties of a minimally invasive restorative material based on evidence-based literature are that it should mimic the natural tooth structure in colour and translucency. It should withstand masticatory forces even in high load-bearing areas for the entire duration of service of the restoration. This ideal material should be of considerable longevity and that the margins of this restorative material injunction with the cavo-surface margins should not be detected and they should not deteriorate with function and time14. The surface characteristics of the restoration should be such that their polish and lustre should remain throughout the life of the restoration. It should be able to induce tertiary dentine formation to reduce the chances of secondary caries12.
The bond to enamel, dentin and cementum should not only be mechanical but chemical as well. It should be able not to stop caries activity but also to remineralize active carious lesions. It should be easily repairable and should bond easily to other restorative materials. It should be biocompatible and not elicit an immune response. It should not stain the tooth. It should be easily retrievable10. It should be osteoinductive as well as osteoconductive and it should be inductive for odontoblasts and cementoblasts as well. It should be easily distinguishable from the surrounding tooth structure so that retreatment can be performed easily. It should preferably induce genes associated with osteoblasts and odontoblast growth, differentiation, and it should promote cell to matrix adhesion. It should set in the presence of moisture. It should be sterile, nontoxic, and non-carcinogenic11. There should be no microleakage, significant shrinkage, expansion, and percolation with time. Currently, no biomaterial that fulfils the requirements of minimally invasive dentistry exists.
Bonding to Dental Hard Tissues
Enamel, dentine, and cementum are structurally, histologically, embryologically and functionally different structures and there are significant difficulties in imitating these God made structures by a single restorative material. That is why materials currently available for enamel; dentine and cementum can be reviewed separately in line with the concept of minimally invasive dentistry.
The ideal material to replace enamel in line with the minimally invasive philosophy is composites, which are versatile in their applications. Advantages of composites are that they are aesthetic, easily repairable, bond to the tooth structure; do not rely on mechanical retention from undercuts, pins, dovetails and grooves and minimal teeth preparation. Disadvantages include polymerization shrinkage, marginal leakage, percolation difference in coefficient of thermal expansion and discolouration with time15. Materials to bond and replace cementum are mostly conventional glass ionomers and resin-modified glass ionomers as composites do not bond to cementum due to less organic substrate available.
Dentin Replacement Dental Biomaterials
Minimally invasive material to replace dentin would be glass ionomer cements (GIC), composites, Mineral Trioxide Aggregate (MTA), bioactive glasses and novel material, which is marketed as dentin replacement material by the name of Biodentine16. Advantages of GIC include adhesion to enamel and dentin and release of fluoride, calcium and aluminium ions and the effect of rechargeability which means uptake of fluoride from the environment and release of fluoride when the fluoride concentration is low in the immediate environment of the GIC 17. While traditionally glass ionomers have been used in lamination or sandwich techniques to replace the bulk of dentine with composites on top to bond to enamel, but recently Biodentine has been advocated which is considered to be more biomimetic and bioactive than glass ionomers cements18.
Materials to replace and bond to cementum have traditionally been conventional glass ionomers cements but resin-modified glass ionomer cements have, also, been advocated especially in sandwich techniques and cervical lining techniques19. Clinical uses of dental biomaterials used in MID are depicted in Figure 1. Biodentine is a very versatile dental restorative material as it can be used for a variety of clinical uses such as dentine replacement, external and internal resorption, direct and indirect pulp capping and as a retrograde and orthograde filling material20. It is perhaps the only restorative material that can be used in endodontics and restorative dentistry, that is why it has to comply with two ISO standards 6876 of dental root canal sealing materials and ISO 9917-1 of water-based cements21.
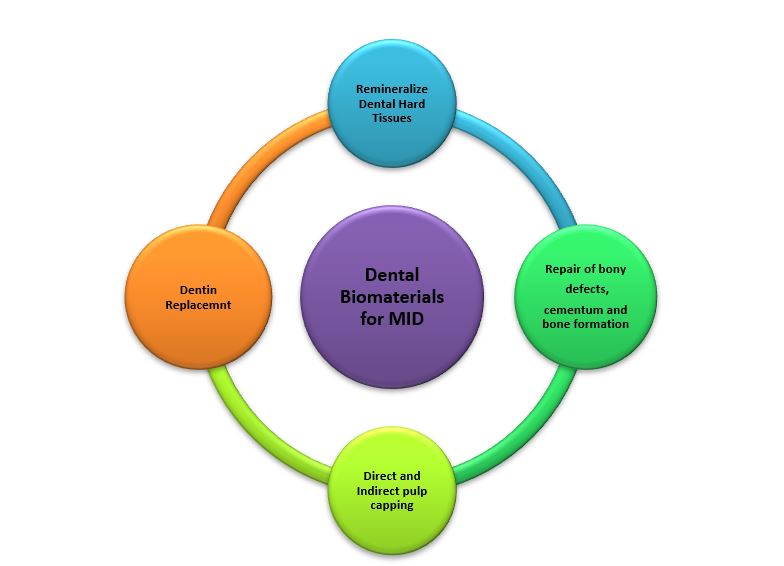
Figure 1: Clinical uses of dental biomaterials for minimally invasive dentistry (MID).
Chemistry of Biodentine
Elucidating the chemistry of Biodentine is paramount to understanding the bioactivity of Biodentine as the final products formed are responsible for the bonding, strength, antimicrobial activity, and induction of odontoblasts18,22,23. The final products formed are dependent on the particle size, shape and distribution of the initial constituents of Biodentine24. The setting or hardening reaction starts with the incorporation of water as each of the components of Biodentine undergo hydration, which contributes to the final product. Calcium silicate and calcium carbonate which acts as fillers contribute to strength25. Tricalcium silicate is responsible for the initial strength and hardening of Biodentine whereas dicalcium silicate is present as a by-product as it is virtually impossible to produce pure tricalcium silicate, which contributes to strength at later times 26. The equation for the hydration of tricalcium silicate, which is the main constituent of Biodentine, is given by:

When water is added, tricalcium silicate also known as alite and dicalcium silicate known as belite react to release calcium ions, hydroxide ions and heat as this is an exothermic reaction27. The pH rises to twelve due to the release of hydroxyl ions, which is responsible for the antimicrobial action of Biodentine. This initial reaction then slows down which results in the reduction of the heat evolved28.The reaction then continues, producing calcium and hydroxide ions until the system becomes saturated29. The next stage then starts in which the calcium hydroxide starts to crystallize. At the same time, calcium silicate hydrate or CSH phase begins to form30. The evolution of heat is then reduced significantly due to calcium and hydroxide ions leaching out of the solution which also shifts the equilibrium of the reaction to the right31. This newly formed calcium hydroxide or portlandite and CSH crystals act as nucleators of crystal growth and they attract more CSH phase to form by aggregation32. This amorphous phase then transforms slowly into a semi-crystalline state due to an increase in thickness of CSH crystals which results in access to water to the hydrated alite and belite33. The diffusion of water molecules through the CSH phase determines the speed of the reaction which slows down the reaction34. This hydration of Biodentine is related to the presence of unbound water and the reaction stops when the unhydrated compounds do not remain in Biodentine35. The rate of hydration is also dependent on temperature as increase in temperature leads to increase in the hydration and setting of Biodentine as long as the increase in temperature is in a humid environment18. The strength of Biodentine is dependent on the formation of silicon tetrahedron bonds and the rate of formation of the CSH phase29.
Since it is a new dental material, that is why research still going on but results so far have been very promising27, 34, 36-42. Merits and problems with Biodentine are given in Table 1
Table 1: Merits and demerits of Biodentine.
| Merits of Biodentine | Clinical problems with Biodentine |
| Bioactive and Biomimetic material | Low compressive strength as compared to other restorative materials |
| Induces tertiary dentine formation43 | Radiopacity low as compared to Mineral Trioxide Aggregate (MTA) |
| Has high initial ph. and has antibacterial action due to it | Needs a two-visit procedure as initial strength is acquired at 48 hours |
| Can be used as a bulk restorative material and as a temporary filling material as well | Long setting and working time although setting time accelerated in a humid environment |
| Interacts with ion exchange and through mineral tag formation in dentinal tubules | Cannot be used as liners as it is not possible to place thin layers |
| Forms strong bond through the formation of the interfacial layer made up of CSH phase 26 | It is relatively expensive as it is cheaper than MTA but more expensive than GICs |
| It favours the release of cytokines and growth factors from the dentin matrix | It is difficult to retrieve it as it forms a strong bond with dentin through the interfacial layer |
| No preliminary conditioning required | ——– |
Material Testing for Minimally Invasive Dentistry (MID) Materials
Testing of bioactive materials follows international and national standards. For a dental restorative to be marketed, it has to satisfy ISO standards, BSI (British Standards International) and ASTM (American Society for Testing and Materials) guidelines, which are considered a bare minimum for it to be approved for marketing. In the United Kingdom, a dental material has to conform to CE markings as well which are considered compulsory for European Economic Area as well44. These material testing requirements for biomimetic and bioactive dental materials have been outlined in Table 2. Test conditions for Biodentine should be at 22 to 23 degrees centigrade and at a relative humidity of 5 to percent and if it is not possible to conduct experiments under such conditions such as measuring compressive strength in Instron machine. Then the samples should be conditioned at this temperature and relative humidity for at least 24 hours before testing45.
Table 2: Recommended material testing for bioactive materials.
| Recommended Material Tests | Description | International Organization for Standardization (ISO)
Standards |
| Microbiological hazard | Claim of material to be non-toxic and free from biological hazards | ISO 10993-1and ISO 74051997 |
| Flow | Should have adequate flow | ISO 6876 clause 7.2 |
| Working time | Should not be less than 90% of the stated time by the manufacturer | ISO 6876 clause 7.3 ISO 6873:1998 |
| Setting time | Should be within the range stated by the manufacturer and should be taken with indenter in moulds | ISO 6876 clause |
| Dimensional change during setting | Should not exceed 1 % in shrinkage and 0.1% in expansion | ISO 6876 clause |
| Solubility | Should not exceed 3% of mass fraction | ISO 6876 clause 7.7 |
| Radiopacity | Should not be less than 3 mm of aluminium | ISO 6876 clause 7.8 |
| Extraneous matter | There should be no extraneous matter | ISO 6876 clause 7.1 |
| Chemical composition and application | Components of powder and liquid should be given | ISO 9917
ISO 6873:1998 |
| Marking, Labelling, Providing Instructions | There should be clear marking on capsules and liquid containers of
manufacturer’s batch and lot number |
ISO 9917, 6876 |
| Acid erosion test | Should be within the thickness in mm given by the manufacturer | ISO 6876 |
| X-ray diffraction | For identification of solid phases and crystalline nature | ——- |
| Acid soluble arsenic and lead content | Should not exceed the relevant limits | ISO 6876 and ISO 9717 |
| Dye leakage test | For sealing ability using methylene blue dye | ——- |
Proposed Modifications to Biodentine
Proposed modifications will possibly alter the chemical composition of Biodentine, and it will transform Biodentine. That is why it will no longer be called Biodentine. The proposed enhancement to Biodentine would be the incorporation of 45S5 Bioglass along with fluoride and strontium with the removal of zirconium oxide46. Colouring agents would also be added to give it a tooth-like appearance. This material would have increased radiopacity over Biodentine due to the presence of strontium ions, would have fast setting and working time due to linkage of silicon tetrahedron with that of 45S5. It would induce the formation of fluorapatite due to the presence of fluoride and it would form hydroxyapatite due to the dual action of bioactive glass and due to the calcium silicate hydrate CSH phase47,48. Furthermore, it will be the ultimate restorative material for use in enamel, dentin and cementum replacement and it would be a tooth coloured restorative due to the presence of colouring agents.
The biological approach of minimally invasive dentistry is dependent on the production and marketing of biomimetic and bioactive dental materials. To overcome the shortcoming of bioactivity and bonding of current restorative materials, a novel restorative “Biodentine” by Septodont was developed and marketed in 2011. The mechanical properties of Biodentine outweigh that of its predecessor MTA but are less than conventional glass ionomers cements. The requirements and guidelines set by different standards for permitting this dental material to be marketed have been outlined. Some drawbacks of Biodentine have been discussed with remedies suggested in the form of development of another bioactive dental material incorporating fluoride, strontium, and bioactive glass with tricalcium silicate and calcium carbonate. This novel material will have superior mechanical and bioactive properties than Biodentine with the potential to make great innovation and contribution to the field of minimally invasive dentistry.
The authors would like to acknowledge the reviewers for providing the valuable feedback on the review.
The authors declare no conflict of interest.
SJAZ did the Conceptualization and proof reading of the review. SK performed the literature search, compilation of material testing requirements and proof reading.
- Clark DJ. Is all minimally invasive dentistry better dentistry? Dent Today. 2009;28(5):98-100.
- Cousley RR, Gibbons AJ. Correction of the occlusal and functional sequelae of mandibular condyle fractures using orthodontic mini-implant molar intrusion. J Orthod. 2014;41(3):245-253.
- Araujo E, Perdigão J. Anterior veneer restorations – an evidence-based minimal-intervention perspective. J Adhes Dent. 2021;23(2):91-110.
- Terrer E, Koubi S, Dionne A, Weisrock G, Sarraquigne C, Mazuir A, et al. A new concept in restorative dentistry: light-induced fluorescence evaluator for diagnosis and treatment. Part 1: diagnosis and treatment of initial occlusal caries. J Contemp Dent Pract. 2009;10(6):86-94.
- Wei Y, Xu T, Hu W, Zhao L, Wang C, Chung KH. Socket preservation following extraction of molars with severe periodontitis. Int J Periodontics Restorative Dent. 2021;41(2):269-275.
- Shiffman HS, Khorsandi J, Cauwels NM. Minimally-Invasive combined Nd:YAG and Er:YAG laser-assisted uvulopalatoplasty for treatment of obstructive sleep apnea. Photobiomodul Photomed Laser Surg. 2021;39(8):550-557.
- Mackenzie L, Banerjee A. The minimally invasive management of early occlusal caries: a practical guide. Prim Dent J. 2014;3(2):34-41.
- Lam R. Minimally legally invasive dentistry. Aust Dent J. 2014;59(4):432-438.
- de Almeida Neves A, Coutinho E, Cardoso MV, Lambrechts P, Van Meerbeek B. Current concepts and techniques for caries excavation and adhesion to residual dentin. J Adhes Dent. 2011;13(1):7-22.
- Huysmans MC, Roeters FJ, Opdam NJ. Cariology and restorative dentistry: old and new risks. Ned Tijdschr Tandheelkd. 2009;116(6):291-297.
- Kielbassa AM, Muller J, Gernhardt CR. Closing the gap between oral hygiene and minimally invasive dentistry: a review on the resin infiltration technique of incipient (proximal) enamel lesions. Quintessence Int. 2009;40(8):663-681.
- Blum IR, Özcan M. Reparative dentistry: possibilities and limitations. Curr Oral Health Rep. 2018;5(4):264-269.
- Duplantis C. Conservation of tooth structure utilizing bonded hybrid ceramics. Compend Contin Educ Dent. 2021;42(2):78-84.
- Khan SI, Asghar S, Abid A, Aftab F. Awareness regarding minimally invasive dentistry among dentists of Karachi. J Bahria Univ Med Dent Coll. 2019;9(4):294-298.
- Davidson CL, de Gee AJ. Light-curing units, polymerization, and clinical implications. J Adhes Dent. 2000;2(3):167-173.
- Ghafoor S. Bioactive glass: regeneration in critical size defects in tibia of animal models. J Pak Dent Assoc. 2019;28(03):136-142.
- Barata TJ, Bresciani E, Mattos MC, Lauris JR, Ericson D, Navarro MF. Comparison of two minimally invasive methods on the longevity of glass ionomer cement restorations: short-term results of a pilot study. J Appl Oral Sci. 2008;16(2):155-160.
- Atmeh AR, Chong EZ, Richard G, Boyde A, Festy F, Watson TF. Calcium silicate cement-induced remineralisation of totally demineralised dentine in comparison with glass ionomer cement: tetracycline labelling and two-photon fluorescence microscopy. J Microsc. 2015;257(2):151-160.
- Bossù M, Mancini P, Bruni E, Uccelletti D, Preziosi A, Rulli M, et al. Biocompatibility and antibiofilm properties of calcium silicate-based cements: an in vitro evaluation and report of two clinical cases. Biology (Basel). 2021;10(6):1-18.
- Song W, Li S, Tang Q, Chen L, Yuan Z. In vitro biocompatibility and bioactivity of calcium silicate‑based bioceramics in endodontics. Int J Mol Med. 2021;48(1):1-22.
- Lyapina M, Yaneva-Deliverska M, Deliversky J, Kisselova A. European and international standards on medical devices for dentistry. J IMAB–Ann Proc Sci Papers. 2015;21(1):713-717.
- Esteki P, Jahromi MZ, Tahmourespour A. In vitro antimicrobial activity of mineral trioxide aggregate, biodentine, and calcium-enriched mixture cement against Enterococcus faecalis, Streptococcus mutans, and Candida albicans using the agar diffusion technique. Dent Res J. 2021;18.
- Mori GG, Teixeira LM, de Oliveira DL, Jacomini LM, da Silva SR. Biocompatibility evaluation of biodentine in subcutaneous tissue of rats. J Endod. 2014;40(9):1485-1488.
- Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C. Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater. 2015;13(1):43-60..
- Al-Sherbiny IM, Farid MH, Abu-Seida AM, Motawea IT, Bastawy HA. Chemico-physical and mechanical evaluation of three calcium silicate-based pulp capping materials. Saudi Dent J. 2021;33(4):207-214.
- Taylor HFW. Cement chemistry. 2nd ed. London: Thomas Telford; 1997. xviii 459 p.
- Utneja S, Nawal RR, Talwar S, Verma M. Current perspectives of bio-ceramic technology in endodontics: calcium enriched mixture cement – review of its composition, properties and applications. Restor Dent Endod. 2015;40(1):1-13.
- Wang X, Sun H, Chang J. Characterization of Ca3SiO5/CaCl2 composite cement for dental application. Dent Mater. 2008;24(1):74-82.
- Setbon HM, Devaux J, Iserentant A, Leloup G, Leprince JG. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent Mater. 2014;30(12):1291-303.
- Dawood AE, Manton DJ, Parashos P, Wong RH, Palamara JE, Stanton DP, et al. The physical properties and ion release of CPP-ACP-modified calcium silicate-based cements. Aust Dent J. 2015;60(4):434-444.
- Kim JR, Nosrat A, Fouad AF. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J Dent. 2015;43(2):241-247.
- Villat C, Tran XV, Tran VX, Pradelle-Plasse N, Ponthiaux P, Wenger F, et al. Impedance methodology: A new way to characterize the setting reaction of dental cements. Dent Mater. 2010;26(12):1127-1132.
- Khatib MS, Devarasanahalli SV, Aswathanarayana RM, Das P, Nadig RR. Comparison of the sealing ability of Endocem mineral trioxide aggregate and Endoseal mineral trioxide aggregate as a furcal perforation repair material under the operating microscope: An in-vitro Endodontology. 2019;31(1):25-28.
- Tziafa C, Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Dentinogenic responses after direct pulp capping of miniature swine teeth with Biodentine. J Endod. 2014;40(12):1967-1971.
- Raju VG, Venumbaka NR, Mungara J, Vijayakumar P, Rajendran S, Elangovan A. Comparative evaluation of shear bond strength and microleakage of tricalcium silicate-based restorative material and radioopaque posterior glass ionomer restorative cement in primary and permanent teeth: an in vitro J Indian Soc Pedod Prev Dent. 2014;32(4):304-310.
- Camilleri J, Laurent P, About I. Hydration of biodentine, theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod. 2014;40(11):1846-1854.
- De Rossi A, Silva LA, Gatón-Hernández P, Sousa-Neto MD, Nelson-Filho P, Silva RA, et al. Comparison of pulpal responses to pulpotomy and pulp capping with biodentine and mineral trioxide aggregate in dogs. J Endod. 2014;40(9):1362-1369.
- Samyuktha V, Ravikumar P, Nagesh B, Ranganathan K, Jayaprakash T, Sayesh V. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J Conserv Dent. 2014;17(5):467-470.
- Nagas E, Cehreli ZC, Uyanik MO, Vallittu PK, Lassila LV. Effect of several intracanal medicaments on the push-out bond strength of ProRoot MTA and Biodentine. Int Endod J. 2016; 49(2):184-188.
- Topçuoğlu HS, Kesİm B, Düzgün S, Tuncay O, Demİrbuga S, Topçuoğlu G. The effect of various backfilling techniques on the fracture resistance of simulated immature teeth performed apical plug with biodentine. Int J Paediatr Dent. 2015;25(4):248-254.
- Abbas A, Kethineni B, Puppala R, Birapu UC, Raghavendra KJ, Reddy P. Efficacy of mineral trioxide aggregate and biodentine as apical barriers in immature permanent teeth: a microbiological study. Int J Clin Pediatr Dent. 2020;13(6):656-662.
- Khetarpal A, Chaudhary S, Talwar S, Verma M. Endodontic management of open apex using Biodentine as a novel apical matrix. Indian J Dent Res. 2014;25(4):513-516.
- Jeevani E, Jayaprakash T, Bolla N, Vemuri S, Sunil CR, Kalluru RS. Evaluation of sealing ability of MM-MTA, Endosequence, and biodentine as furcation repair materials: UV spectrophotometric analysis. J Conserv Dent. 2014;17(4):340-343.
- Hawsawi RA, Miller CA, Moorehead RD, Stokes CW. Evaluation of reproducibility of the chemical solubility of dental ceramics using ISO 6872: 2015. J Prosth Dent. 2020;124(2):230-236.
- ISO 6876:2012. Geneva, Switzerland. 2012.
- Brauer DS, Karpukhina N, O’Donnell MD, Law RV, Hill RG. Fluoride-containing bioactive glasses: effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010;6(8):3275-3282.
- Salako N, Joseph B, Ritwik P, Salonen J, John P, Junaid TA. Comparison of bioactive glass, mineral trioxide aggregate, ferric sulfate, and formocresol as pulpotomy agents in rat molar. Dent Traumatol. 2003;19(6):314-320.
- Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. 2015; 41(3):409-411.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
