By Faiza Zeeshan1, Muhammad Sabir Waheed2, Fakhur Uddin3, Bahram Khan4, Vinita Kumari1, Binish Arif Sultan1
AFFLIATIONS:
- Department of Pathology, Sindh Medical College, Jinnah Sindh Medical University, Karachi, Pakistan.
- Department of Pathology, Karachi Medical and Dental College, Karachi, Pakistan.
- Department of Microbiology, Basic Medical Sciences Institute, Jinnah Post Graduate Medical Centre, Karachi, Pakistan.
- Department of Dermatology, Jinnah Post Graduate Medical Centre, Karachi, Pakistan.
Background: Tinea capitis (TC) is a fungal infection that victimizes every age group. The fundamental culprits of TC are dermatophytes and the role of non-dermatophytes (NDM) in pathogenesis is overshadowed. Therefore, the current study was designed to evaluate the epidemiology of non-dermatophytes as the etiologic agent of tinea capitis among local population.
Methods: It was a cross sectional descriptive study, which was conducted at the Department of Microbiology, Jinnah Post Graduate Medical Centre Karachi Pakistan, from January 2019 to September 2019. A total of 207 patients diagnosed with tinea capitis were enrolled in the study. The scalp scrapings and hair were collected and processed for Potassium Hydroxide (KOH), Lactophenol Cotton Blue (LPCB) and Calcofluor White (CFW) staining. The specimens were cultured on Dermatophyte Test Medium (DTM) and Sabouraud Dextrose Agar (SDA). The species were identified by slide culture, LPCB staining and biochemical tests. The Chi squared test was used for determining the association between variables. The kappa index was utilized for determining the correlation between the efficacies of tests, provided that p-value lesser than 0.05 was considered statistically significant.
Results: Among isolated species, 61(29.5%) were dermatophytes and 45(21.7%) were non-dermatophytes. The most common isolated non-dermatophytes were Aspergillus spp. (n=16, 35.5%), followed by Penicillium spp. (n=7, 15.55%). A significant association was observed between the non-inflammatory type of lesions of TC and non-dermatophytes (p-value=0.000). CFW staining was found to be a better tool in detecting fungal components in the specimen compared to KOH mounts (p-value=0.000).
Conclusion: The non-dermatophytes carry substantial importance in causing tinea capitis and related superficial scalp mycoses.
Keywords: Aspergillus; Dermatophytes; Tinea Capitis; Epidemiology.
Tinea capitis (TC) is a dermatophytic scalp infection, which affects hair, and its contiguous skin1. The school-aged children are commonly infected and so as the adolescents, but its incidence in adults is sporadic2. It is approximated that 92% of childhood mycoses are solely related to TC 3. The infection is seen significantly in resource-constrained communities of developing and developed regions and is easily transmissible among family members by sharing common household items. The infection can be non-inflammatory to inflammatory and becomes further complicated by superimposed bacterial infections. The non-inflammatory form is associated with seborrhea, hair loss, and pruritus. On the other hand, pustules, tender plaques with broken hair, characterize inflammatory TC. The clinical presentation of TC often mimics bacterial folliculitis, alopecia areata, seborrheic dermatitis, and scalp psoriasis, making its diagnosis ambiguous3,4.
The etiologic agents of TC are dermatophytes, which are keratinophilic moulds and are considered as the primary offending agent. The dermatophytes produce a variety of proteolytic enzymes, which ease hair penetration and pathogenesis. The ability of dermatophytes to adhere to the keratin containing cells is related to fungal dimorphism, temperature sensing, production of collagenases and elastases5. The genes SUB1, SUB2, and SUB3 are identified in dermatophytes, which are related with serine encoding proteases, which aid in fungal adherence and peneteration6,7. All these factors in collaboration determine the virulence of dermatophytes.
The role of dermatophytes in TC is indubitably inevitable, but pathogenesis by non-dermatophyte moulds and yeasts is blocked out particularly in the context of TC and scalp mycoses. The non-dermatophyte moulds and yeasts have been associated with onychomycosis in certain geographic locations8-10. The involvement of non-dermatophytes (NDM) with TC is recently started to be evaluated, and it is found that non-dermatophytes also play a significant role in causing TC9. Their identification as a causative agent bears utmost importance, as filamentous fungi are not susceptible to various antifungal agents like azoles and flucytosine; hence, ambivalent diagnosis leads to prolong treatment spells and the emergence of recalcitrant and resistant strains. The NDMs are distributed frequently in natural commodities of air, soil, and water.
The ubiquitous presence of various non-dermatophytes makes humans and animals vulnerable to contract non- dermatophytoses. Aspergillus, Curvularia, Alternaria, Scopulariopsis, Penicillium, Mucor, Rhizopus and Rhodotorula spp. are the NDM moulds, which are found to be pathogenic, and Candida spp., are among NDM yeasts. The spores of NDM are resistant, hydrophobic, and higher stress tolerant, therefore can be easily transmitted from one type of environmental condition to another10. The pathogenesis of non-dermatophytes is multifactorial. It ranges from the production of mycotoxins, collagenases, proteases and glycosyl hydrolases to stress signaling pathway activation related to stress response and adaptation11-13.
The prevalence of NDM among scalp infections can vary from region to region. Studies from Iran, Saudi Arabia, Brazil, Bulgaria and Ethiopia have elucidated the involvement of non-dermatophyte moulds and yeasts in dermatophytoses but exclusive research on non-dermatophytes, in context to scalp mycoses is still limited11. In Pakistan, the prevalence of TC among various age groups is found to be 13.6% to 30.3%14-16. The major offending agent involved is Trichophyton violaceum, but data regarding non-dermatophytes is not sufficient17. In addition, the difference in treatment options for non-dermatophyte moulds and yeasts gives them diagnostic importance. Therefore, the study aimed to determine the epidemiology and role of non-dermatophyte moulds and yeasts as an etiologic agent in TC among the local population.
The study was conducted at the Department of Microbiology, Basic Medical Sciences Institute in collaboration with the Department of Dermatology of Jinnah Post Graduate Medical Centre, Karachi Pakistan after getting approval from Institutional Review Board (F-2-81/2019-GENL/33047/JPMC). The study design was descriptive cross sectional and sample size was determined by using an OpenEpi menu, available online. The study was carried out on 207 individuals, which were referred from the dermatology outpatient department with a presumptive diagnosis of tinea capitis by a simple random technique. The patients of both genders undescriptive of age were included, however; subjects who were using or had used topical or systemic antifungal agents in the past two weeks were excluded for enrollment.
The data regarding age, gender, socioeconomic status, co-morbid diseases, presenting complaints, drug and family history, contact with animals, duration of illness, and history of recurrence were recorded in a pre-designed questionnaire. The signs and symptoms were carefully assessed and verified by a dermatologist, which were simultaneously recorded in the same questionnaire. Additional notes were made wherever required.
The hair along with its contiguous skin scales was collected on filter paper by using sterilized pair of forceps and glass slide, respectively. One part of the specimen was processed for Potassium hydroxide (KOH) mount and Calcofluor white (CFW, Sigma-Aldrich) staining while the other part was inoculated on Dermatophyte Test Medium (DTM) and Sabouraud Dextrose Agar (SDA, Oxoid UK). For each sample, six vials were inoculated (two for each media) and were incubated at 25°C and 30°C15,16. The specimens were considered positive for fungal components if they showed fungal spores and hyphae on KOH mount or produced apple green fluorescence with CFW on fluorescent microscope. The inoculated samples were monitored daily for two weeks for the presence of fungal colony, pigmentation, and pigmentation on the reverse. The samples were said to be culture positive when 3 out of 6 vials showed growth within four weeks and those samples were declared culture negative, which failed to show significant growth after four weeks. The isolated colonies were subcultured on Potato Dextrose Agar (PDA) and slide culture, Urease test, and Lactophenol Cotton Blue (LPCB) staining was performed for species identification. The data were recorded and analyzed on IBM Statistical Package for Social Sciences (SPSS) version 20. The cross tabulations and Chi squared test were used for determining the association between variables. The kappa index was utilized for determining the correlation between the efficacies of tests with p<0.05 was considered statistically significant.
Out of 207 individuals, 115(55.6%) were males and 92(44.4%) were females. The mean age was found to be 12.74±11.15 years with a minimum reported age of 1 year and maximum age of 61 years. The disease was prevalent in the lower socioeconomic class, around 196(94.7%) subjects belonged to low income group while 10(4.8%) were from middle-income strata and only 1(0.5%) belonged to high income group. The family history of similar lesions was observed in 35(16.9%) patients and 30(14.5%) study participants mentioned the sharing of common household objects within family members as shown in Table 1.
Table 1: Clinical attributes of study population.
| Clinical Attributes | Children (≤12 years) (n=114) |
Adolescents (12-21 years) |
Adults (>21 years) (n=40) |
Total (n=207) |
p-Value | |
| Gender distribution |
Male Female |
62 | 29 | 24 | 115 | 0.06 |
| 50 | 25 | 17 | 92 | |||
|
Duration of illness (Mean ±SD) |
Male Female |
3.3±0.2 | 4.6±0.6 | 5.4±1.0 | 4.51±0.9 | 0.03 |
| 3.7±0.7 | 3.9±1.1 | 6.2±1.6 | ||||
| History of recurrence |
Male Female |
12 | 7 | 3 | 39 | 0.33 |
| 9 | 6 | 2 | ||||
| Cases with co-morbidity |
Male Female |
1 | 2 | 5 | 13 | 0.10 |
| 1 | 1 | 3 | ||||
| Family history of similar lesions on scalp |
Male Female |
10 | 6 | 3 | 35 | 0.68 |
| 8 | 5 | 3 | ||||
| Sharing of household items |
Male Female |
8 | 3 | 2 | 30 | 0.81 |
| 8 | 6 | 3 | ||||
The use of herbal remedies and oil was observed in 76(36.7%) study participants and a significant association was found between the culture positivity for non dermatophytes and application of oil and herbal remedies (p=0.03). The inflammatory and non-inflammatory lesions were observed in 107(51.7%) and 100(48.3%) participants, respectively. The non-inflammatory lesions were significantly associated with non-dermatophytes and inflammatory lesions were predominantly seen with dermatophytes (p=0.000). The commonest presenting complaint was patchy alopecia, observed in 141(68.1%) subjects.
Among 207 samples, 106(51.2%) were culture positive and 101(48.8%) were culture negative. Out of culture positive, 61(57.54%) were dermatophytes and 45(42.45%) were non-dermatophyte moulds and yeasts. Aspergillus spp. constituted about 16(35.5%) of all non-dermatophytes. The Aspergillus niger 9(20%) was the most common isolated species among Aspergillus group followed by Aspergillus fumigatus 4(8.8%), Aspergillus terreus 2(4.4%) and Aspergillus flavus 1(2.2%) as illustrated in Figure 1.
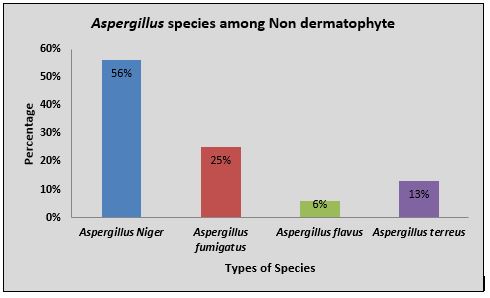
Figure 1: Distribution of Aspergillus species among non-dermatophyte moulds and yeasts.
The Penicillium spp. 7(15.5%), Cladosporium 5(11.1%) Curvularia spp. 6(13.3%) and Alternaria spp. 6(13.3%) were isolated in 24(39.9%) cases of non-dermatophytes respectively. The Candida albicans was isolated in 3(6.6%) cases, while Mucor and Scopulariopsis were seen in 1(2.2%) case each as shown in Figure 2.
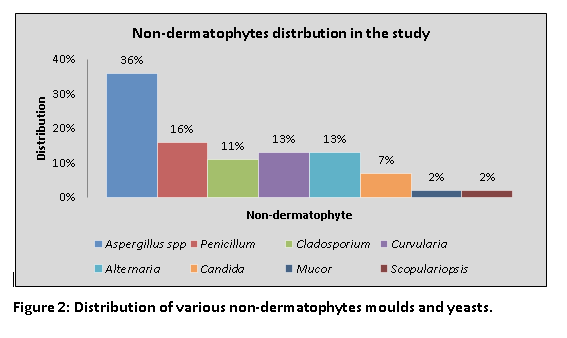
Figure 2: Distribution of various non-dermatophytes moulds and yeasts.
About 31(68.8%) cases of non-dermatophytes gave fungal positivity on KOH mount. The four patterns of hair invasion were found on KOH mount: ectothrix, endothrix, favus and mixed. The most common pattern of hair invasion observed in non-dermatophyte positive cases was mixed 16(31.25%). The CFW staining technique revealed fungal elements in 44(97.7%) cases of culture positive cases for non-dermatophyte moulds and yeasts. The CFW staining was found to be an excellent tool in demonstrating the fungal spores and hyphae in specimens (p-value=0.000). The kappa index showed moderate agreement between the KOH mount and CFW staining (k=0.415) as shown in Table 2.
Table 2: Correlation between potassium hydroxide (KOH) mount, calcoflour white (CFW) staining and mycological culture.
| Tests | Fungal positive cases n (%) | p-Value | Correlation | Kappa Index | Level of agreement |
| KOH | 32(72.1) | 0.001 | Between KOH and CFW staining | 0.415 | Moderate agreement |
| CFW Staining | 44(97.7) | 0.000 | Between KOH and culture | 0.0098 | Slight agreement |
| Culture | 45(42.4) | 0.000 | Between CFW staining and KOH | 0.008 | Disagreement |
*p-value<0.05 is considered significant; **<0= disagreement, 0.01–0.20 Slight agreement, 0.41–0.60 Moderate agreement18
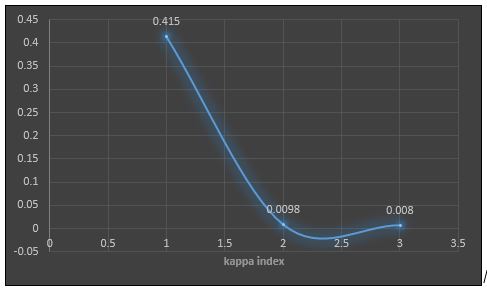
Figure 3: Correlation between Direct microscopic techniques and culture.
Our study highlighted the role of non-dermatophyte moulds and yeasts in the etiology of Tinea capitis (TC). Superficial mycoses of the scalp in general and TC, in particular, are linked closely with dermatophytes. The dermatophytes are related conventionally to TC, but the involvement of non-dermatophyte moulds and yeast is often ignorant and under rated. The current study highlighted the presence of non-dermatophyte moulds and yeast as a causative agent of TC. According to our study, Aspergillus niger was the commonest offender among the NDM category as well as among Aspergillus group. The Aspergillus flavus was isolated in 9(20%) cases of total NDM followed by Aspergillus fumigatus 4(8.8%). This result was in full union with Kaur et al. and partial concurred with Person et al. who declared Aspergillus flavus as the second most common isolate among Aspergillus group19,11.
The Aspergillus terreus and Aspergillus flavus were positive in 2(4.4%) and 1(2.2%) case respectively. Among, non-Aspergillus group, the most common isolated fungal species were Curvularia and Alternaria which were identified in 6(13.3%) cases of NDM. The Cladosporium was also isolated in 5(11.1%) cases and Candida albicans was positive in 3(6.66%) specimens. The Mucor and Scopulariopsis have seen in 1(2.2%) case each. The presence of Candida albicans is related largely to the immune status of the body and is considered an opportunistic pathogen. Interestingly, we documented the isolation of Candida Albicans from immunocompetent individuals. These results approved with a case report by Fortuna et al. who also reported involvement of Candida Albicans in immunocompetent patient20.
The yielding of NDM moulds and yeasts from hair samples signify the importance of culture and related laboratory tests. The KOH mount is the basic diagnostic tool, which can detect the presence of fungal hyphae and spores in a given sample. By KOH, we observed fungal positivity for NDM in 31(68.8%) cases. A mixed pattern of hair invasion on KOH was observed in maximum cases of NDM i.e., 16(35.5%). The pattern of hair invasion was said to be mixed when fungal spores and hyphae were visualized both in and out of the hair shaft. CFW staining is another convenient technique, which produces reliable results. In our study, the sensitivity of CFW staining to detect NDM was found to be 93.2%. CFW staining was found to be a better diagnostic tool for NDM compared to dermatophytes (p-value=0.000). The kappa index for correlation between KOH mount and CFW staining was calculated as k=0.415 which indicated moderate degree of agreement between the two tests. The culture was considered as a gold standard technique in our study. However, culture was positive in 45(42.45%) cases of NDM. Our results were similar to the results of Janardhan and Vani who reported KOH mount as a more sensitive method for diagnosing NDM in comparison with culture21. A study by Bonifaz et al. declared positivity for KOH in 66.6% cases, culture in 33.33% and CFW in 57.58% cases 22. This study further reinforces our documented findings. Our study strongly supports the utility of simple and easy laboratory methods for detecting possible fungal pathogens especially in resource-poor settings where molecular techniques for diagnosis are not available.
Our data showed that the mean age of study subjects was found to be 12.74±11.15 years and was predominantly observed in the male population (55.6%, p=0.02). This established that prepubescent children are more prone to contract a fungal infection of the scalp. The higher rates of TC among young teens are related primarily to the decreased amount of saturated fatty acids in sebum, which possess fungistatic properties. The increased level of androgens and testosterone in males after puberty exerts a protective mechanism against fungal infections by increasing the amount of fungistatic C7-C11 saturated fatty acids in sebum, making male adolescents and adults less vulnerable to be victimized by fungal infections23. Another reason for male preponderance is related to the social setup observed in Pakistan where males are supposed to be involved in outdoor chores more often than females, therefore environmental exposure to fungal spores is higher in males in comparison with females. Another stipulated reason in literature is the presence of Pityrosporum orbiculare, which colonizes the scalp at the age of puberty and infringes dermatophyte invasion, therefore prepubertal children are more commonly affected24.
TC is considered as a disease of poverty and is largely related to the economic wellbeing of the family and community. Our study showed similar findings, being common in a lower socio-economic group (94.7%). Due to the paucity of resources, the sharing habit of common household articles among family members was observed in 14.5% of subjects. The use of the same combs, hairbrushes, pillows, caps, head veils etc, facilitated the transference of fungal spores from one person to another. Hence, 16.9% of patients gave a history of similar lesions among family members. Kalu et al., Chikoi et al. and Adesiji et al. also reported similar epidemiological conditions in their studies, which included the involvement of low socio-economic class, sharing of common household objects and positive family history25-27. The application of herbal oil and other remedies to curb dandruff and associated scalp disorders are in common practice in Pakistan. The current study highlighted the association of oil application on scalp and culture positivity for non-dermatophytes (p=0.03). Viswanath et al. reported similar results of association of oil application on scalp and fungal infection28. Although saturated fatty acids of oil are said to be fungistatic our results were contradictory. This contradiction might be related to the unhygienic practices of study subjects including infrequent head washing which can aggravate seborrhea and pruritus. The non-inflammatory lesions were observed in 48.3% patients and were significantly associated with non-dermatophytes. The most common presenting complaint was patchy alopecia, noted in 68.1% subjects. Patchy alopecia is an annoying problem and can lead to emotional and social distress in patients therefore, it was the major reason for patients to visit outpatient’s department of dermatology. Our results were in accordance with Veasey and Muzy while Attal et al. reported seborrheic type as the most common complaint in non-inflammatory TC29,30.
Different non-dermatophytes species were isolated from the patients of Tinea capitis (TC). The demographic attributes have also highlighted the contagious pattern of disease along with its possible modes of transmission. The clinicians and dermatologists should consider the NDM moulds and yeasts as a causative agent of TC and diagnosis should be confirmed by conventional and contemporary methods.
The authors would like to acknowledge the hospital facility for supporting the study.
The authors did not declare any conflict of interest.
The Institutional Review Board (IRB) of Jinnah Post Graduate Medical Centre approved the study with the letter number (F-2-81/2019-GENL/33047/JPMC).
The consents were taken from patients before the data collection procedures.
FZ contributed to study conceptualization, design, data collection and manuscript writing. SW analyzed the data, critically reviewing it. FU performed data analysis, helped in manuscript writing and literature search. BK helped in data collection, critical reviewing and manuscript writing. BA contributed in data collection and data analysis and VK assisted in data analysis and critical reviewing.
- Gupta AK, Mays RR, Versteeg SG, Piraccini BM, Shear NH, Piguet V, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32(12):2264-2274.
- Chokoeva AA, Zisova L, Chorleva K, Tchernev G. Aspergillus niger–a possible new etiopathogenic agent in Tinea capitis? Presentation of two cases. Braz J Infect Dis. 2016;20(3):303-307.
- Bassyouni RH, El-Sherbiny NA, Abd El Raheem TA, Mohammed BH. Changing in the epidemiology of tinea capitis among school children in Egypt. Ann Dermatol. 2017;29(1):13-19.
- Ely JW, Rosenfeld S, Stone MS. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90(10):702-710.
- Park MW, Hong JS, Suh MK, Ha GY, Jang TJ, Choi JS. Tinea capitis caused by Microsporum canis misdiagnosed as seborrheic dermatitis in a 79-year-old woman. Korean J Med Mycol. 2017;22(3):129-134.
- John AM, Schwartz RA, Janniger CK. The kerion: an angry tinea capitis. Int J Dermatol. 2018;57(1):3-9.
- Martinez-Rossi NM, Peres NT, Rossi A. Pathogenesis of dermatophytosis: sensing the host tissue. Mycopathologia. 2017;182(1-2):215-227.
- Maddy AJ, Abrahams JL, Tosti A. Onychomycoses due to non-dermatophytic molds. Onychomycosis. 2017: 61-71. Springer, Cham.
- Bitew A. Dermatophytosis: prevalence of dermatophytes and non-dermatophyte fungi from patients attending Arsho advanced medical laboratory, Addis Ababa, Ethiopia. Dermatol Res Pract. 2018;2018:1-7.
- Motamedi M, Ghasemi Z, Shidfar MR, Hosseinpour L, Khodadadi H, Zomorodian K, et al. Growing incidence of non-dermatophyte onychomycosis in Tehran, Iran. Jundishapur J Microbiol. 2016;9(8):1-6.
- Person AK, Chudgar SM, Norton BL, Tong BC, Stout JE. Aspergillus niger: an unusual cause of invasive pulmonary aspergillosis. J Med Microbiol. 2010;59(7): 834-838.
- Paulussen C, Hallsworth JE, Álvarez‐Pérez S, Nierman WC, Hamill PG, Blain D, et al. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus Microb Biotechnol. 2017;10(2):296-322.
- Hagiwara D, Sakamoto K, Abe K, Gomi K. Signaling pathways for stress responses and adaptation in Aspergillus species: stress biology in the post-genomic era. Biosci Biotechnol Biochem. 2016;80(9):1667-1680.
- Jehangir F, Vohra EA. Frequency of tinea capitis in children 5-15 years of age presenting to primary health care centre in Karachi, Pakistan. Infect Dis J Pak. 2013;22(3)592-595.
- Hussain A, Zakki SA, Qureshi R. Epidemiological study of dermatophytosis in Okara, Pakistan. Res Rev J Pharm Pharm Sci. 2016;4(1):184-187.
- Ahmed I, Ahmed Z, Nasreen S. Prevalence of tinea capitis and asymptomatic carriage amongst school going children. J Pak Assoc Dermatol. 2017;16:215-219.
- Araya S, Tesfaye B, Fente D. Epidemiology of dermatophyte and non-dermatophyte fungi infection in Ethiopia. Clin Cosmet Investig Dermatol. 2020;13: 291-297.
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360-363.
- Kaur R, Panda PS, Sardana K, Khan S. Mycological pattern of dermatomycoses in a tertiary care hospital. J Trop Med. 2015;2015:1-6.
- Fortuna MC, Garelli V, Pranteda G, Carlesimo M, D’Arino A, Rossi A. Scalp infection by Candida Albicans in an immunocompetent patient: a description of a rare case. J Chemother. 2018;30(5):316-317.
- Janardhan B, Vani G. Clinico mycological study of dermatophytosis. Int J Res Med Sci. 2017;5(1):31-19.
- Bonifaz A, Rios-Yuil JM, Arenas R, Araiza J, Fernández R, Mercadillo-Pérez P, et al. Comparison of direct microscopy, culture and calcofluor white for the diagnosis of onychomycosis. Rev Iberoam Micol. 2013;30(2):109-111.
- Jo JH, Deming C, Kennedy EA, Conlan S, Polley EC, Ng WI, et al. Diverse human skin fungal communities in children converge in adulthood. J Investig Dermatol. 2016;136(12):2356-2363.
- Venitarani SA, Handayani S, Ervianti E. Profile of patients with tinea capitis. Dermatol Rep. 2019;11(1)62-64.
- Kalu EI, Wagbatsoma V, Ogbaini-Emovon E, Nwadike VU, Ojide CK. Age and sex prevalence of infectious dermatoses among primary school children in a rural South-Eastern Nigerian community. Pan Afr Med J. 2015;20(1):1-12.
- Chikoi R, Nyawale HA, Mghanga FP. Magnitude and associated risk factors of superficial skin fungal infection among primary school children in Southern Tanzania. Cureus. 2018;10(7):1-12.
- Adesiji YO, Omolade BF, Aderibigbe IA, Ogungbe OV, Adefioye OA, Adedokun SA, et al. Prevalence of tinea capitis among children in Osogbo, Nigeria, and the associated risk factors. Dis. 2019;7(1):13-22.
- Viswanath V, Kriplani D, Miskeen AK, Patel B, Torsekar RG. White piedra of scalp hair by Trichosporon inkin. Indian J Dermatol Venereol Leprol. 2011;77(5):591-593.
- Veasey JV, Muzy GD. Tinea capitis: correlation of clinical presentations to agents identified in mycological culture. An Bras Dermatol. 2018;93(3):465-466.
- Attal RO, Deotale V, Yadav A. Tinea capitis among primary school children: a clinicomycological study in a rural hospital in central India. Int J Curr Res Rev. 2017;9(23):25-31.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
