By Mehreen Inam Illahi1, Sofia Amjad1, Syed Mehfooz Alam2, Syed Tousif Ahmed1, Murk Fatima1
AFFLIATIONS:
- Department of Physiology, Ziauddin University, Karachi, Pakistan.
- Department of Rheumatology, Liaquat National Hospital, Karachi, Pakistan.
Background: Rheumatoid arthritis (RA) is a progressive inflammatory disease affecting the joints with a marked impact upon functional capacity of the patient. The working ability of RA patients can be preserved if the disease modifying antirheumatic drug (DMARD) therapy is initiated early in the course of disease. The objective of our study was to compare the disease activity variables in DMARD-naïve seropositive rheumatoid arthritis (SPRA) and seronegative rheumatoid arthritis (SNRA) patients and to determine correlations between the disease activity variables in RA.
Methods: A cross-sectional study recruited n=90 patients from Rheumatology Clinic from May 2020 to October 2020. The rheumatoid factor (RF), anti-cyclic citrullinated peptide levels (ACCP), erythrocyte sedimentation rates (ESR) were clinically measured. Disease activity variables including the tender joint count (TJC), swollen joint count (SJC), health assessment questionnaire-disability index (HAQ-DI) and disease activity score of 28 joints (DAS28) were consistently calculated. Patients were divided into seropositive RA group and seronegative RA group, based on RF and ACCP. Chi squared test and Pearson correlation were applied, p≤0.05 was considered statistically significant.
Results: High HAQ-DI and DAS28-ESR scores were found in SPRA than in the SNRA patients and were statistically significant (p=0.000, p=0.054). TJ-28 and SJ-28 counts were higher in SPRA but were not statistically significant. There was a significant correlation of DAS28 with TJ-28 (r=0.816, p-value = 0.000), with SJ-28(r=0.801, p-value = 0.000) and HAQ-DI (r=0.517, p-value = 0.000).
Conclusion: Evaluation of inflammatory markers and functional disability was found significant (p=0.000) in determining the disease activity compared to presence of autoantibodies in DMARD naïve RA patients.
Keywords: Disease Modifying Antirheumatic Drug; Arthritis; Rheumatoid Factor; Autoimmune Diseases.
Rheumatoid arthritis (RA), an immune mediated disease predominantly causes inflammation and progressive decrease in joint function. It affects 0.24% population globally. Females are more affected by RA than males1,2. Its prevalence in Pakistan is reported to be 0.5%. Thus, it has a pronounced impact upon working ability of the patient and causes complete disability in many patients, which forces them to quit their jobs3. Life expectancy of RA patients is reported to be significantly reduced due to aggressiveness of this chronic inflammatory arthritis particularly in the first few years of the diagnosis.
The evaluation of serum autoantibodies: rheumatoid factor (RF) and anti cyclic citrullinated peptide (ACCP) in RA is an important tool in confirming the diagnosis especially in those with an unclear clinical picture. Among these autoantibodies, ACCP provides a more specific evidence of RA diagnosis. Based on these serum autoantibodies as mentioned in 2010 American College of Rheumatology/European League against Rheumatism (ACR/EULAR) criteria for RA has enabled the stratification of RA patients as seropositive (SPRA) and seronegative rheumatoid arthritis (SNRA) patients4.
The targeted treatment approach to prevent functional disability in RA patients requires optimal measurement of disease activity. The evaluation of severity of disease in RA is done by several indices including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and disease activity score (DAS).The disease activity is well monitored by ESR over a couple of weeks while CRP shows short-term changes in disease activity of RA5.
Clinically Disease Activity Score for 28 joints (DAS28) was previously taken as DAS, is officially recommended by EULAR to evaluate disease activity6. DAS-28 is a well-balanced combined measure of disease activity from 28 swollen joints (SJ-28), 28 tender joints (TJ-28), ESR/CRP and general health of patient. DAS28 can range from 0 to 10, with scores of, >5.1 (high disease activity), <3.2 (low disease activity) and <2.6 (remission) 7. SpecificallyDAS28-ESR is widely used in prognosis and treatment monitoring in RA patients and it has been suggested by Aletaha et al. that DAS28-ESR of 2.4 more suitably reflects remission in RA8,9.
Functional disability of RA patients is evaluated by patient based health assessment questionnaire-disability index (HAQ-DI). It includes questions regarding the patient’s functional status. Total score ranges between 0–3 10. A delay in identifying RA in early part of the disease can cause significant joint inflammation and damage to the joints. Initiation of disease modifying anti-rheumatic drugs (DMARD) has been shown to reduce advancement in joint inflammation in early RA11. Hence, if the disease activity in RA is monitored and well controlled with the use of DMARDs at appropriate time then joint deformity can be prevented and mobility of joints can be well preserved. This study was done to evaluate the working disability in RA patients so that DMARD therapy can be initiated at the start of disease symptoms. The objective of our study was to compare the disease activity variables in DMARD-naïve SPRA and SNRA patients and to determine correlations between the disease activity variables in RA.
A total of 90 patients from 30 to 65 years with the complaint of arthralgia were recruited from Rheumatology Clinic. It was a cross-sectional study conducted from May 2020 to October 2020 after an approval from Ethical Review Committee of Ziauddin University (Ref code: 0920319MIPHY). The 2010 American College of Rheumatology/European League against Rheumatism (ACR/EULAR) criteria was used to diagnose RA in participants of this study. All of these patients were DMARD naïve. All participants gave an informed consent and filled in a questionnaire about the performance of their activities in daily life as assessed by the Stanford University HAQ-DI (Health Assessment Questionnaire-disability index)12 which included 20 questions in eight domains: (1) dressing and grooming (2) arising (3) eating (4) walking (5) hygiene (6) reach (7) grip and (8) common daily activities. The responses of patients were measured as scores from 0 to 3, mentioned in HAQ-DI: 0=able without any difficulty, 1=able with some difficulty, 2=able with much difficulty and 3=unable. A certified trained rheumatologist did clinical examinations. The total HAQ score was calculated as given in HAQ-DI. The DAS28—including 28 tender (TJ28) and swollen joint (SJ28) count and the erythrocyte sedimentation rate (ESR)—was used to assess clinical disease activity by a DAS28-ESR calculator13.
Venous blood sample of 5ml was taken from each patient by a trained phlebotomist and it was centrifuged at 3000 rpm to get the serum and stored in multiple aliquots at -80˚C. RF levels were determined using a rheumatoid factor (RF) enzyme linked immunosorbent assay (ELISA) Kit (MBS721682) (MyBioSource, San Diego, California, USA) according to the manufacturer protocol. The reference range was from 5.0-100IU/mL and analytical sensitivity was 1.0IU/mL, ACCP levels were determined using an anti-cyclic citrullinated peptide (ACCP) antibody, enzyme linked immunosorbent assay (ELISA) kit (MBS720363) (MyBioSource, San Diego, California, USA) according to the manufacturer protocol. The reference range was from 25-500U/mL and analytical sensitivity of 1.0U/mL Patients were included in seropositive RA group because of presence of RF/ACCP both or alone RF or ACCP.
Statistical package social sciences version 20 was used for statistical analysis. Frequencies and percentages were analyzed for categorical variables. Mean and standard deviation (SD) were analyzed for numerical variables. Comparison of two groups was done with the use of independent t-test. For finding correlation between variables, Pearson correlation was applied, p-value of 0.05 was considered as significant.
A total of 90 patients diagnosed with RA were recruited for this study of which 76(84.4%) were females and 14(15.6%) were males. Among the 90 patients, 68(75.6%) had seropositive whereas 22(24.4%) had SNRA. Of the 68 seropositive RA patients, 40 (58.8%) were positive for both RF and ACCP, 20(29.4%) were positive for ACCP, 8(11.7%) were positive for RF. Our data showed high levels of ACCP than the RF in seropositive RA group. The Table 1 shows the comparison of demographic and anthropometric characteristics and disease activity variables of SPRA and SNRA patients. The mean values of height (p=0.012) were significantly different in both subgroups of RA patients while age and other anthropometric variables were not significantly different.
Table 1: Demographic and anthropometric characteristics of rheumatoid arthritis (RA) patients included in the study.
| Demographic and Anthropometric Characteristics | |||
| Variable | Seropositive RA
(n=68) |
Seronegative RA
(n=22) |
p-Value |
| Age (Mean-SD) | 45.63±10.16 | 48.63 ±9.32 | .223 |
| Weight (kg) | 63.05±7.15 | 60.90±3.00 | .175 |
| Height (m) | 1.69±.06 | 1.65±.07 | .012* |
| BMI (kg/m²) | 22.4±2.64 | 22.81±1.96 | .517 |
| Disease Activity Variables in RA Patients | |||
| ESR mm/hr | 46.49 ±33.22 | 26.59 ±12.08 | .007* |
| TJ-28 | 10.08 ±4.98 | 8.63 ±6.80 | .283 |
| SJ-28 | 6.42 ±4.82 | 5.81 ±6.44 | .638 |
| HAQ.DI | 1.48 ±.532 | 1.18 ±.501 | .000* |
| DAS-28 | 5.39 ±.984 | 4.92±1.00 | .054* |
| BMI, body mass index, DAS28, 28 joint disease activity score; ESR, erythrocyte sedimentation rate; HAQ-DI, health assessment questionnaire disability index, SD, standard deviation | |||
It was observed that there was a significant difference in mean ESR values, between the two groups SPRA, 46.49±33.22: SNRA, 26.59±12.08 mm/hr and was statistically significant (p=0.007). Mean HAQ.DI was higher in SPRA (1.48 ±.532) than in the SNRA (1.18±0.501) patients and was statistically significant (p=0.000). DAS-28 was reported to be higher in SPRA (5.39±0.984) than in SNRA (4.92±1.00) group and was found to be significant (p=0.054). TJ-28 and SJ-28 counts were found to be higher in SPRA, (10.08 ±4.98 and 6.42 ±4.82) than in the SNRA group (8.63±6.80 and 5.81±6.44) but were not shown to be statistically significant.
Table 2: Comparison of disease activity score of 28 joints (DAS28) among the rheumatoid arthritis (RA) groups.
| Rheumatoid Arthritis (RA) Groups | Low
(<2.6) |
Moderate
(3.21-5.1) |
High
(>5.1) |
p-Value | |
| Seropositive Rheumatoid Arthritis (n=68) | n | 5 | 26 | 37 | 0.00 |
| % | 7.4 | 38.2 | 54.4 | ||
| Seronegative Rheumatoid Arthritis (n=22) | n | 1 | 17 | 4 | |
| % | 4.5 | 77.3 | 18.2 | ||
Table 2 shows the comparison of DAS28 in the two RA sub-groups. The Chi-squared test was applied to see the association of DAS28 among these groups. It was observed that among the seropositive RA, 7.4% had low disease activity, 38.2% had moderate disease activity and 54.4 % had severe disease activity. Among the seronegative RA, 4.5 % had low disease activity, 77.3 % had moderate disease activity and 18.2 % had severe disease activity. The Chi-squared test showed a significant association of DAS28 in the RA groups with p=0.00. Hence, this result showed increased number of patients with high disease activity in seropositive RA group whereas increased number of patients with moderate disease activity in seronegative RA group. In our study a significant correlation was observed between the disease activity variables, DAS28 and HAQ-DI (r=0.514, p-value = 0.000), shown in Figure 1.
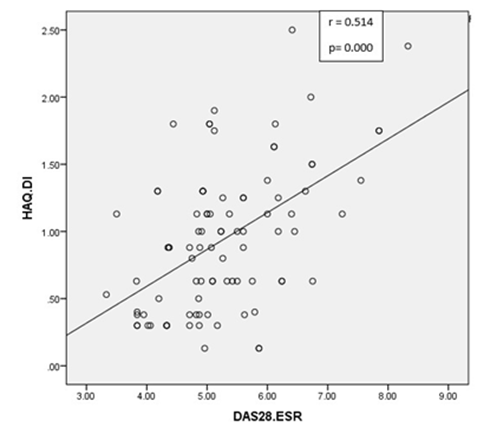
Figure 1: Correlation between Disease Activity Score in 28-Joints (DAS-28) and Health Assessment Questionnaire Disability Index (HAQ-DI).
In our study, the disease activity was compared in two groups of DMARD naïve SPRA and SNRA patients. Our study has reported high disease activity in seropositive RA patients as reflected by high DAS-28. This was may be due to the high ESR levels in SPRA patients in our study. These same findings were reported by other studies11,14,15. In our study, greater number of patients was reported to have high disease activity scores in seropositive RA group and in seronegative RA group; greater number of patients were found to have moderate disease activity. However, Choi and Lee reported that disease activity was higher in seronegative RA patients16. High 28 TJC and 28 SJC were seen in SPRA patients. Papadopoulos et al. also reported in his study that SPRA group had higher 28 TJC and 28 SJC17,18. The high disease activity in SPRA patients was probably caused by the presence of autoantibodies, RF and ACCP in SPRA patients that leads to joint deformities and therefore causes functional disability in these patients19. An ACCP-containing immune complex (ACCP-IC) triggers the release of inflammatory cytokine TNFα via Fcγ R-dependent pathway by macrophages20, 21. These cytokines attack the synovium in joints of RA patients and leads to an aggressive disease and ultimately results in joint erosion22. However, Nordberg et al. has reported significantly high SJC in seronegative RA patients4. Another study also reported same finding that 28 TJC and 28 SJC components were significantly higher in SNRA versus the SPRA group16. This variation in disease outcome in subsets of RA patients may be attributed to the differences in inclusion criteria, genetics and disease activity variables assessed among these studies.
Functional disability as assessed by HAQ-DI was less in SNRA group. This is due to the low disease activity in these patients. A cohort study on RA patients reported similar findings15. In contrast to our study, Nordberg et al. reported that DAS28 was significantly higher in seronegative RA as compared to seropositive RA patients4,16.
Our data showed a significant correlation of DAS-28 with HAQ-DI as also seen in another study23. Association of DAS28 and HAQ-DI was also done in another study to evaluate disease activity in RA24. van der Heijde et al., and Boyd et al. mentioned that there is an association of HAQ-DI and DAS-28 in SPRA patients25,11. This is because of the cytokines that damage the synovium, which causes joint inflammation and deformity, hence altering the functional ability in these patients.
Our study also reported that levels of ACCP were more than the RF in seropositive RA group. Similar findings were reported in other studies26, 27. ACCP assays offer a slight advantage over RF due to its higher specificity26,27,28. This is may be due to the high inflammatory activity in majority of the patients’ positive for ACCP. Therefore, ACCP assay can be more valuable as compared to RF in identifying RA patients29. Our study was a single centered study thus, future multiple centers studies should be done on a large number of patients. In addition to this a follow up study to monitor progress of disease, should be conducted to make necessary alterations in the treatment in both subgroups of RA patients.
Evaluation of inflammatory marker ESR, DAS28 and HAQ-DI are considered to be more significant in determining the disease activity than the presence of auto antibodies in DMARD naïve Rheumatoid Arthritis patients. Hence, patients with arthralgia coming to rheumatology clinic should be followed up regularly every month by evaluating inflammatory markers and determining functional disability particularly in SNRA population who may show clinical signs only but remain undiagnosed because of the absence of RF and ACCP leading to a delay in their diagnosis.
We would like to thank the Ziauddin University for the financial support and Dr. Syed Mehfooz Alam (Consultant Rheumatologist). We are also thankful to Mr. Moazzam Ali for his support in the laboratory work.
The authors declare no conflict of interest.
The Ethics Review Committee of the Ziauddin University approved the study.
Verbal and written informed consents were obtained from all the patients.
MII had given the concept of study, written the manuscript and performed the basic lab work. SA had drafted the write up and revised it critically. SMA collected the data and STA critically evaluated it. MF performed the data analysis and interpretation.
- Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316-1322.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham III CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
- Akhter E, Bilal S, Haque U. Prevalence of arthritis in India and Pakistan: A review. Rheumatol Int. 2011;31(7):849-855.
- Nordberg LB, Lillegraven S, Lie E, Aga AB, Olsen IC, Hammer HB, et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naïve patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis. 2017;76(2):341-345.
- Firestein GS. Kelley’s textbook of rheumatology. Philadelphia, PA: Saunders. Elsevier; 2009.
- Siemons L. Measuring disease activity in patients with early rheumatoid arthritis [dissertation]. University of Twente; 2014.
- Van der Heijde DM, Van’t Hof MA, Van Riel PL, Van Leeuwen MA, Van Rijswijk MH, Van de Putte LB. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis. 1992;51(2):177-181.
- Porter D, Gadsby K, Thompson P, White J, McClinton C, Oliver S. DAS28 and rheumatoid arthritis: the need for standardization. Musculoskeletal Care. 2011;9(4):222-227.
- Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625-2636.
- Yousefi H, Chopra A, Farrokhseresht R, Sarmukaddam S. Clinical assessment and health status in standard care in Indian and Iranian patients suffering from rheumatoid arthritis (RA). Indian J Rheumatol. 2014;9(2):57-61.
- Boyd TA, Bonner A, Thorne C, Boire G, Hitchon C, Haraoui B, et al. The relationship between function and disease activity as measured by the HAQ and DAS28 varies over time and by rheumatoid factor status in early inflammatory arthritis (EIA). Results from the Catch Cohort. Open Rheumatol J. 2013; 7: 58-63.
- The Stanford Health Assessment Questionnaire: Stanford University School of Medicine, Division of Immunology and Rheumatology. 2005.
- DAWN Visual. DAS 28 Calculator [Internet]. DAS 28 – Disease Activity Score Calculator for Rheumatoid Arthritis; 2020 [updated 10/08/2020; cited 2020 Dec 5]. Available from: http://www.4s-dawn.com/DAS28/
- Chalan P, Bijzet J, Van Den Berg A, Kluiver J, Kroesen BJ, Boots AM, et al. Analysis of serum immune markers in seropositive and seronegative rheumatoid arthritis and in high-risk seropositive arthralgia patients. Sci Rep. 2016;6(1):1-9.
- Mouterde G, Rincheval N, Lukas C, Daien C, Saraux A, Dieudé P, et al. Outcome of patients with early arthritis without rheumatoid factor and ACPA and predictors of rheumatoid arthritis in the ESPOIR cohort. Arthritis Res Ther. 2019;21(1):1-9.
- Choi ST, Lee KH. Clinical management of seronegative and seropositive rheumatoid arthritis: a comparative study. PLoS One. 2018;13(4):1-10.
- Papadopoulos I, Katsimbri P, Katsaraki A, Temekonidis T, Georgiadis A, Drosos A. Clinical course and outcome of early rheumatoid arthritis. Rheumatol Int. 2001;20(5):205-210.
- Coffey CM, Crowson CS, Myasoedova E, Matteson EL, Davis III JM. Evidence of diagnostic and treatment delay in seronegative rheumatoid arthritis: missing the window of opportunity. Mayo Clin Proc. 2019; 94(11): 2241-2248.
- Salma K, Nessrine A, Krystel E, Khaoula EK, Noura N, Khadija E, et al. Rheumatoid arthritis: Seropositivity versus seronegativity; a comparative cross-sectional study arising from moroccan context. Curr Rheumatol Rev. 2020;16(2):143-148.
- Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, et al. Induction of macrophage secretion of tumor necrosis factor α through Fcγ receptor IIa engagement by rheumatoid arthritis–specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58(3):678-688.
- Laurent L, Clavel C, Lemaire O, Anquetil F, Cornillet M, Zabraniecki L, et al. Fcγ receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann Rheum Dis. 2011;70(6):1052-1059.
- Seegobin SD, Ma MH, Dahanayake C, Cope AP, Scott DL, Lewis CM, et al. ACPA-positive and ACPA-negative rheumatoid arthritis differ in their requirements for combination DMARDs and corticosteroids: secondary analysis of a randomized controlled trial. Arthritis Res Ther. 2014;16(1):1-12.
- Ghosh A, Ghosh B, Pain S, Pande A, Saha S, Banerjee A, et al. Comparison between DAS28, CDAI and HAQ-DI as tools to monitor early rheumatoid arthritis patients in eastern India. Indian J Rheumatol. 2011;6(3):116-122.
- Salaffi F, Cimmino M, Leardini G, Gasparini S, Grassi W. Disease activity assessment of rheumatoid arthritis in daily practice: validity, internal consistency, reliability and congruency of the Disease Activity Score including 28 joints (DAS28) compared with the Clinical Disease Activity Index (CDAI). Clin Exp Rheumatol. 2009;27(4):552-559.
- van der Heijde D, Klareskog L, Singh A, Tornero J, Melo-Gomes J, Codreanu C, Pedersen R, Freundlich B, Fatenejad S. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis. 2006;65(3):328-334.
- Aridoğan BC, Kaya S, Savaş S, Cetin ES, Akkuş S, Demirci M. The role of anti-cyclic citrullinated peptide (anti-CCP) antibodies in serologic diagnosis and evaluation of disease activity in rheumatoid arthritis. Mikrobiyol Bul. 2008;42(4):669-674.
- Matsui T, Shimada K, Ozawa N, Hayakawa H, Hagiwara F, Nakayama H, et al. Diagnostic utility of anti-cyclic citrullinated peptide antibodies for very early rheumatoid arthritis. J Rheumatol. 2006;33(12):2390-2397.
- Moghimi J, Ghorbani R, Hasani F, Sheikhvatan M. Discriminative and diagnostic value of anti-cyclic citrullinated peptide antibodies in Iranian patients with rheumatoid arthritis. Rheumatol Int. 2013;33(3):601-605.
- Esmat MM, Moghazy HM, Boghdady AM, Hassan A. Anti-cyclic citrullinated peptide (ACCP) antibody versus rheumatoid factor (RF) for diagnosis of rheumatoid arthritis. Egypt J Med Microbiol. 2012;38(1231):1-6.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
