By Kausar Aamir1, Arfa Azhar1, Fatima Abid2, Aliya Jafri2, 3, Noorulain Qureshi2, Sumaira Riffat2
AFFILIATIONS:
- Department of Biological and Biomedical Sciences, Aga Khan University Hospital (AKUH).
- Department of Physiology, Jinnah Sindh Medical University.
- Department of Biochemistry, Jinnah Sindh Medical University, Karachi, Pakistan.
ABSTRACT
Background: Preeclampsia is a multifactorial disorder leading to complications of pregnancy. Vitamin E as an antioxidant has been proved to suppress the oxidative stress mechanism by interfering with the propagation of lipid radicals. The objective of the study was to probe antioxidant nutrient profiles in patients with a risk factor for preeclampsia.
Methods: This study was done in the Obstetric Outpatient Department of Jinnah Postgraduate Medical Centre, Karachi from 2019-2019. The study group comprised 110 patients divided into three groups as Group A: n=40 Normotensive patients, Group B: n=40 Mild hypertensive (test group I), Group C: n=30 Severe hypertensive (test group II). SPSS version 21 was used for data analysis with p-value 0.05 considered as statistically significant.
Results: Plasma ascorbic acid levels stayed considerably (p<0.001) decreased in both mild cases group B mean+SD (0.36±0.02 mg/ dl) and severe cases group C (0.3±0.02 mg/ dl) of pregnancy-induced hypertension (PIH) women, while matched with the normal expecting women group A (0.49±0.28 mg/d1). Serum alpha-tocopherol (vitamin E) (0.32±0.00 mg/dl) and beta-carotene (0.04±0.002 mg/ dl) levels were significantly reduced with p values (p<0.001) highly significant to normal pregnant women (control) p<0.001 and highly significant compared to mild cases of pregnancy-induced hypertensive women. Results also showed Alpha-tocopherol or vitamin E (0.74±0.03) and beta-carotene (0.129±0.00 mg/dl) levels matched with group A. The values of serum Vitamin A in all three groups remains significant.
Conclusion: Adequate antioxidant nutrients have a utility in preventing free radical-mediated cell disruptions (p=0.001) in patients at risk of preeclampsia by protecting endothelial cell damage.
Keywords: Hypertension; Pregnancy Induced Hypertension; Oxidative Stress; Antioxidants.
Preeclampsia (PE) affects approximately 7-10% of all pregnancies. It is concomitant with improved perinatal ill health and mortality associated with intrauterine growth impedance premature delivery and perinatal asphyxia. Furthermore, pregnant women stand at increased risk for abruption placenta, intracerebral hemorrhage, and hepatic and renal shutdown1. Vascular endothelial hurt is recognized to show a part in the pathophysiologic etiology of preeclampsia2.
Endothelial cell damage is believed to decrease prostacyclin formation, ensuing in an increase in peripheral vascular resistance and platelet aggregation3. However, the pathogenesis of such endothelial cell damage remains unclear. It has been reported that free radical-mediated lipid peroxidation may be affected by endothelial dysfunction observed in preeclampsia4. Several justifications support this notion, together with a proliferation in lipid peroxidation products and a drop-in antioxidant activity in preeclampsia as matched with normal gestation5. Antioxidant protects cell membranes from free radical-induced lipid peroxidation. Additional free radical instabilities are classically complemented by improved consumption of antioxidants6.
Gathering confirmation from clinical and epidemiologic studies proposes that diffuse endothelial dysfunction occasioning from oxidative stress shows a central part in preeclampsia’s pathophysiological basis7. Human plasma comprises an assortment of low molecular weight, non-enzymatic antioxidants that assist in looking after the vasculature from oxidative damage2 and nitrogen species3. In addition, ascorbic acid can spare or recover glutathione and vitamin E (α-tocopherol) two other vital physiologic antioxidants. In the red to these important characteristics of antioxidant investigators have theorized that ascorbic acid influence prevents or ameliorates oxidative stress prompted endothelial dysfunction and preeclampsia4.
Women with pre-eclampsia (PE) have a little concentration of antioxidant vitamins. They reinforce the concept of increased oxidative stress. The early supply of antioxidants, such as vitamins C and E, to women at high risk of developing PE has a significant clinical benefit. The use of vitamins is associated with improved endothelial function and less placental damage. Tocopherols can act as scavengers of free radicals and alpha-tocopherol, particularly in vivo, in this regard8. There is also substantiation for abridged antioxidant status and improved free radical production (oxidative stress) in PE; few antioxidants have been reported to reduce in PE, including this, vitamin E, ascorbate and carotenoids9.
Peroxidic lipids (resulting from lipid oxidation), plasma concentrations of antioxidants, and plasma concentrations of vitamins E and C (antioxidants) equivalent to those of pregnant women are normal in women with PE10. Women with PE had reduced antioxidant activity, which supports the idea that lipid peroxidation by free radicals and associated antioxidant consumption may play a role in the pathophysiological mechanism of preeclampsia. Free radicals can diffuse into the cell membranes that cause lipid peroxidation, the propagation of which can be suppressed by alpha-tocopherol5. Vitamin E and C supplementation in pregnant women at high risk of PE was helpful for PE inhibition11. Therefore, the objective of the study was to probe antioxidant nutrient profiles in patients with a risk factor for preeclampsia. The study also aimed to investigate and compare antioxidant nutrient profiles in patients with different grades i.e., mild, and severe pregnancy-induced hypertension with normal pregnant controls.
A total of n=200 gravid women got their perinatal care scheduled over Obstetric OPD of Jinnah Postgraduate Medical Centre (JPMC), Karachi, was enlisted, after informed on paper consent, from September 2018 through September 2019. Subjects age 15-38 years, gestational age 20-42 weeks, and single-term pregnancy was included. A small epidemiologic form was completed by each participant related to personal history, maternal age, weight, height, systolic and diastolic blood pressure readings, parity, gestational age, any underlying illness, drug abuse history, family history of PIH (pregnancy induced hypertension) or any complication occurred in previous pregnancies, etc. Recent dietary intake was also recorded. Women having a history of vitamin supplementation before pregnancy, having prolonged hypertension (either essential or next to renal disease, endocrine disease, anemia, malnutrition, mal-absorption, or other diseases), multiple pregnancies and abnormal levels of serum creatinine (i.e., 1.5 mg/dl) were omitted from the study. All procedures were in accordance with the ethical standards of Helsinki’s declaration after approved by ethical committee of Basic Medical Sciences Institute, JPMC Karachi, Pakistan (Ref. No.F.1-2-/2018/BMSI-E COMT/071/JPMC).
The Institutional Review Panel sanctioned the study procedure. Identification of pre-eclampsia was established in accordance with the delineations of “The American College of Obstetricians and Gynecologists (ACOG)”. Pre-eclampsia remained categorized mild least one or more of the subsequent signs or indications were existing wherein situation the pre-eclampsia was ordered as severe8. Systolic blood pressure ≥160 mmHg or 110 mmHg, diastolic taking place two occasions 6 hours apart, Oliguria in 24 hours (urinary output < 400 to 500 ml), cerebral or optical instabilities, pulmonary edema, or cyanosis, epigastric or right upper quadrant pain, the Impaired liver function of uncertain source or thrombocytopenia was monitored in all patients7,8.
Out of 200 patients, 90 patients did not follow up until the end of the study period due to unexplained reasons. Finally, 110 patients were followed and divided in three groups: Group A (n=40) Normotensive patients (control group) i.e., pregnant women > 20 weeks gestation. Group B (n=40) Mild hypertensive > 140/90 mmHg (test group I) i.e., pregnant women > 20 weeks gestation. Group C (n=30) Severe hypertensive > 160/110 mmHg (test group II) i.e., pregnant women > 20 weeks gestation. Fasting venous blood testers were collected in heparin-coated tubes. Plasma was parted by centrifugation and initially kept at -80ºC to be analyzed later but within one week. The α-tocopherol level was tested using means of HPLC (high-pressure liquid chromatography). High-performance liquid chromatography is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material causing different flow rates for the different components and leading to the separation of the components as they flow out of the column. A and B-carotene and vitamin C were analyzed by Micro techniques of clinical chemistry by Samuel Natelson Platelet count was done by fractional centrifugation after citric acid blood coagulation9,10. A 24-hour urine sample was collected for proteinuria estimation by the urine dipstick method.
Statistical software SPSS version 21 was used for data feeding and analysis. A descriptive statistical analysis of continuous variables was performed. Data on continuous variables i.e., biophysical, and biochemical parameters were presented as mean+standard deviation (SD). All the parameters were statistically evaluated by the Chi-square test after the study period. A p-value<0.05 was considered statistically significant.
The mean maternal age of the severe cases of pregnancy-induced hypertensive was (mean±SD) 20.33±0.77 years which was significantly (p<0.01) low as compared to mild cases i.e., 23.37±0.75 years and highly significant while linked to the control cases i.e., 25.0±0.6 years. The mean weight of normal pregnant women was 60.4±0.5 Kg, while the mean weight of the mild and severe cases of pregnancy-induced hypertensive women was 58.05±0.4 Kg and 56.6±0.36 Kg (Table 1) respectively.
Table 1: Clinical parameters of normal pregnant and pregnancy induced hypertensive women.
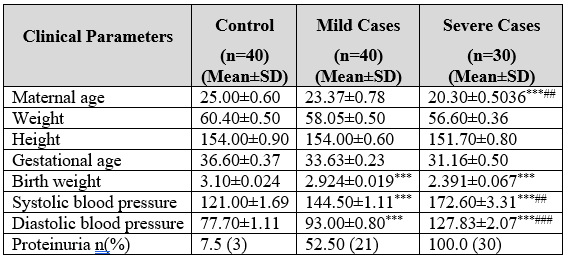
*** (p<0.001) highly important as associated with normal pregnant women (control) ### (p<0.001) highly important as associated with mild cases of pregnancy-induced hypertensive women. ** (p<0.01) Significant as associated with normal pregnant women (control). ## (P<0.01) Significant as associated with mild cases of pregnancy-induced hypertensive women. *** (p<0.001) highly important as likened to normal pregnant women (control) ### (p<0.001) highly important as likened to mild cases of pregnancy induced hypertensive women.
There was no increase in the mean weight of pregnancy-induced hypertension (PIH) cases compared to the control, because of their comparative lower gestational ages, which were (mean±SD) 33.63±0.23 and 31.16±0.5 weeks in mild and severe cases of pregnancy-induced hypertension. The mean gestational age of the controls was 36.6±0.37 weeks. The mean height of all respective groups was non-significant. The mean birth weight of mild cases was 2.924±0.019 Kg, and the severe group was 2.391±0.067 Kg; cases were highly expressively diminished (p<0.001) as compared to birth weight of normal pregnant controls i.e., 3.100±0.024.
The increase in systolic (144.5±1.11 mmHg) and diastolic (93.0±0.8 mmHg) blood pressure of mild cases and systolic (172.6±3.31 mmHg) and diastolic (127.5±2.07 mmHg) of severe cases were extremely noteworthy (p<0.001) (Table 1) when associated to controls: systolic (121±1.69 mmHg) and (77.7±1.11 mmHg). The rise in the systolic and diastolic blood pressure values for severe cases (group C) were again very important (p<0.001) when compared with the mild cases of PIH. Proteinuria was detected in 3 (7.5%) cases of normal pregnant women, 21 (52.5%) cases of mild PIH and 30 (100%) cases of severe PIH women.
Serum alpha-tocopherol (vitamin E) (Table 2) was significantly low in severe and mild cases (0.32±0.00 mg/dl, 0.74±0.03 mg/dl respectively), when compared with normal pregnant women levels (0.78±0.040).
Table 2: Comparison of the antioxidant nutrient profile among normal pregnant and pregnancy induced hypertensive women.
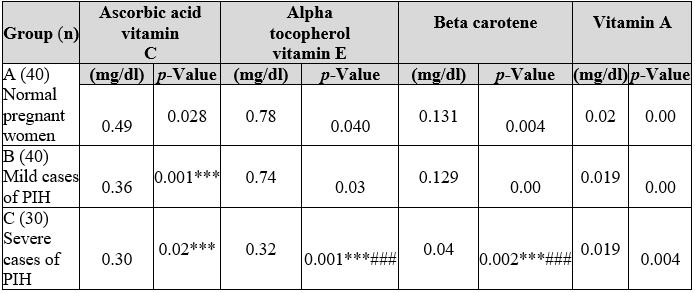
*** (p<0.001) highly significant as matched to normal pregnant women (control). ### (p<0.001) highly significant as matched to mild cases of pregnancy-induced hypertensive women. Showed Alpha-tocopherol or vitamin E (0.74±0.03) and beta-carotene (0.129±0.00 mg/dl) while matched with group A or normal expectant women levels. The values of serum Vitamin A in all three groups remains significant.
Table 2 and Figure 1 compare the antioxidant nutrient profile among the three groups A, B and C, respectively. Plasma ascorbic acid levels stayed considerably (p<0.001) decreased in both mild cases group B (0.36±0.02 mg/ dl) and severe cases group C (0.3±0.02 mg/ dl) of PIH women, while matched with the normal expecting women group A (0.49±0.28 mg/d1). Serum alpha-tocopherol (vitamin E) (0.32±0.00 mg/dl) and beta-carotene (0.04±0.002 mg/ dl) levels were significantly reduced. In terms of percentage presence of vitamin C in serum was 42.6% in group A (control) of the total patients in the study. This percentage of vitamin C was reduced as 31.3% in the serum of group B (mild PIH) patients. Further serum reduction of vitamin C as 26.1% was observed in the serum of group C (severe PIH) of the total patients in the study.
Results of vitamin E (a-tocopherol) levels in the serum were observed as 42.4% in group A (control) of the patients in the study. The percentage of serum intensities of vitamin E was decreased to 40.2% in-group B (mild PIH) patients in the study. In contrast, the serum levels of vitamin E remained further decreased to 17.4% in group C (severe PIH) patients. Results were observed as B-carotene levels in the serum were 44.9% in group A (control) patients. The percentage serum levels of 0-carotene were reduced to 41.4% in group B (mild PIH) patients in the study and serum levels of B-carotene were further reduced to 13.7% in group C (severe PIH) patients in the study. Results were observed as vitamin A levels in the serum as 34.5% in group A (control) of patients in the study. The percentage of serum levels of vitamin A was reduced slightly to 32.8% in-group B (mild PIH) patients in the study. The serum levels of vitamin A remained the same as 32.8% in group C (severe PIH patients in the study.
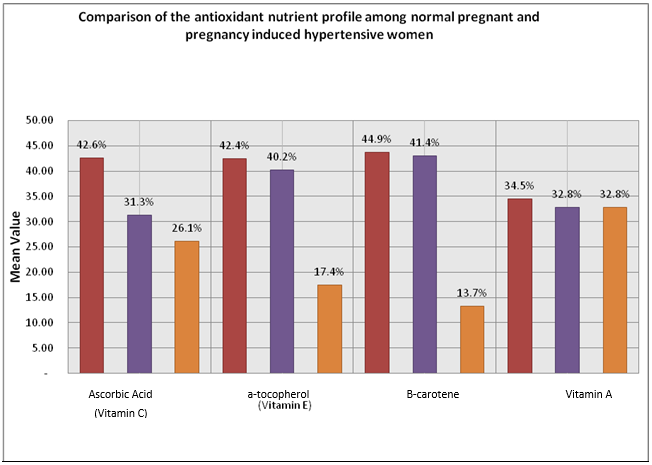
Figure 1: Comparison of the antioxidant nutrient profile among normal pregnant and pregnancy induced hypertensive women.
The main contributor to maternal and fetal death and morbidity is preeclampsia. Oxidative stress has been associated with the pathophysiology of preeclampsia. Falling by the levels of the antioxidant nutrients observed in this study indirectly proposes an idea that hyperexcitation of free radicals mediated lipid peroxidation and the comparative utilization of antioxidants may be involved in the pathophysiological mechanism pregnancy-induced hypertension12. Physiologic irregularities of preeclampsia have been attributed to abnormalities in endothelial cells of maternal vessels due to the emergence of free radicals from the defective placenta13. The main oxidation product of α-tocopherol is a tocopherylguinone, which can be conjugated to give glucuronate after the previous lessening with hydroquinone. It is expelled in the bile or additional degraded in the kidneys to α- tocopheryl acid and thus emitted in the bile. Vitamin E proteins not selected preferentially by the hepatic binding proteins are removed during the secretory process of very-low-density lipoprotein (VLDL) in the liver and probably excreted by the bile. Part of vitamin E can also be excreted by cutaneous sebaceous glands14.
The role of nourishment in usual and unusual gestations has long been discussed. This study focused on plasma/serum concentration and the possible antioxidant role in pregnancy-induced hypertension and hypercholesterolemia. Our results determine that plasma levels of ascorbic acid were expressively reduced in mild and severe patients of pregnancy-induced hypertension, while alpha-tocopherol and beta-carotene levels were meaningfully reduced only in patients with severe cases of pregnancy-induced hypertension. These discoveries are comparable to those distinguished by Al-Gubory15.
Our study showed decrease in vitamin A concentrations in mild and severe cases stayed insignificant despite its substantial singlet oxygen scavenging ability. The possible share of vitamin A and other retinoids in malignancy prevention has however been reviewed recently16. In patients with pregnancy-induced hypertension, we hypothesize that antioxidant nutrients may be used to gain further free radical-induced cells endometrial movement, resulting in a decrease in antioxidant levels. Water-soluble nutrient, the ascorbic acid lipid-soluble antioxidant is seen initially and then followed antioxidants, Tocopherol alpha, and beta-carotene. Several findings are worth mentioning. Initially, smoking is known to reduce plasma antioxidant nutrient concentrations, mostly those of ascorbic acid and beta-carotene in this study, there were no smokers. An antioxidant nutrient level in smaller studies indicates that the independent effects of smoking17.
The concentration of the antioxidant nutrients can be predisposed thru several reasons, as well as nutritional intake. In this study, we cannot complete a nutritive assessment. Some examples may be a solemn assembly to deny that they are all fears of the recent fast blow of nutrient intake because of the chastisement of a union. If it is excluded from the study of patients with abnormally high levels of creatinine, renal function may be the removal of nutrients. People now repeatedly take multivitamin supplements too during pregnancy and women who are excluded, since supplements can be produced to support any issues. In short, the very homogeneous with what they were once equally subject to socioeconomic image of the same region, has recruited a sufficient catchment customer.
The mean maternal age and parity of the severe cases of pregnancy-induced hypertension were significantly low compared to normal pregnancy. Primigravida and teenagers were at a higher risk of developing the disease. There is a sharp rise in incidence above the age of 35 years18,19. The main purpose of selecting control cases with a lower parity was to avoid malnutrition and anemia in such women, which is commonly seen in women with high parity and poor socioeconomic conditions. The present outcomes remain in settlement through a current finding of decreased antioxidant action in females having pregnancy-induced hypertension. In normal gestation, there is an alteration in the percentage of vitamin E to lipid peroxides in maternal blood that gradually favors vitamin E through progressing pregnancy20.
Sarwar et al. in their study also proved that vitamin E levels were unchanged in mild cases of pregnancy-induced hypertension but were significantly decreased in severe cases20. The proportion of lipid peroxides to alpha tocopherol was improved in mild. Besides, it significantly improved in severe cases of pregnancy-induced hypertension. Alpha tocopherol is a free radical scavenger and thus applies antioxidant action. On the other hand, alpha-tocopherol is used in applying its usage, so unusual upsurges in lipid peroxides in pregnancy-induced hypertension would increase consumption, resulting in the increased alpha-tocopherol levels21.
Concentrated ascorbic acid is a bio-available type of ascorbic acid. This works as per the antioxidant is transformed from the reduced to the oxidized pattern. It has been reported that ascorbic acid is a hydrophilic antioxidant act as a first-line antioxidant against oxygen particles, which appear mainly in plasma. Unlikely, alpha-tocopherol and beta-carotene are the hydrophobic antioxidants that can quench oxygen free radicals, which are mainly present in the membrane of lipid cells22. Ascorbic acid and free radicals that entraps a major part of the water is present in the plasma. Since ascorbic acid exceeded the capability of free radicals in the cell membrane through extensive lipid peroxidation, which can be inhibited by the disclosure of alpha-tocopherol and beta-carotene23. Co-antioxidants i.e., ascorbic acid and beta-carotene make alpha-tocopherol as an efficient antioxidant24.
The right levels of vitamin C maintain and strengthen the powerful antioxidant, namely vitamin E. We restrained the concentrations of three powerful antioxidants, ascorbic acids, alpha-tocopherol, and plasma/serum beta-carotene together with vitamin A. Decreasing the levels of the antioxidant nutrients witnessed in this study indirectly sustenance an idea that hyper excitation of free radicals mediated lipid peroxidation and the relative utilization of antioxidants may be involved in the pathophysiological mechanism pregnancy-induced hypertension25. Our findings which were decreased in vitamin C levels in patients with mild and extreme hypertension caused by pregnancy; however, alpha-tocopherol and beta carotene concentrations merely decreased while the disease existed severe suggested that radical antioxidant interactions in pregnancy-induced hypertension might be introduced in the water-soluble part of the plasma. We estimate that the progress can then be transmitted as disease developments to the endothelial cell membrane.
Serum alpha-tocopherol (vitamin E) was significantly low in severe and mild cases when compared with normal pregnant women levels (0.040). An improved understanding of pregnancy-induced hypertension and its management is the main outcome of the study with good control PIH and safe prolongation of pregnancy until term. In Pakistan, severe preeclampsia due to pregnancy-induced hypertension is very common owing to low socioeconomic status and lack of prenatal care.
The authors would like to acknowledge the staff of Jinnah Postgraduate Medical Centre for their support in data gathering.
The authors declare no conflict of interest.
All procedures were in accordance with the ethical standards of Helsinki’s declaration after approved by ethical committee of Basic Medical Sciences Institute, JPMC Karachi, Pakistan (Ref. No.F.1-2-/2018/BMSI-E COMT/071/JPMC).
The written and verbal consents were obtained for the research.
KA presented the idea and reviewed the manuscript; AA did the literature review and FA contributed in critical review and statistical analysis. AJ assisted in research write up, NA helped in analysis and editing and SR performed the review of manuscript.
- Katsiki N, Godosis D, Komaitis S, Hatzitolios A. Hypertension in pregnancy: classification, diagnosis and treatment. Aristotle Univ Med J. 2010;37(2):9-18.
- Chen Q, Sousa JD, Snowise S, Chamley L, Stone P. Reduction in the severity of early onset severe preeclampsia during gestation may be associated with changes in endothelial cell activation: A pathological case report. Hypertens Pregnancy. 2016;35(1):32-41.
- Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med. 2015;21(2):88-97.
- Ardalić D, Stefanović A, Kotur-Stevuljević J, Ninić A, Spasić S, Spasojević-Kalimanovska V, et al. Lipid indexes and parameters of lipid peroxidation during physiological pregnancy. J Lab Med. 2019;43(2):93-99.
- Davis M. Maternal Cholesterol Levels during Pregnancy [ PhD’s thesis]. Michigan State University; 2018. 23 p.
- Mason SA, Della Gatta PA, Snow RJ, Russell AP, Wadley GD. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: findings of a randomized controlled study. Free Radic Biol Med. 2016;93:227-238.
- Hansson SR, Nääv Å, Erlandsson L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front Physiol. 2015;5:516-516.
- Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017;13:94-162.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1): R27-R44.
- Soma-Pillay P, Catherine NP, Tolppanen H, Mebazaa A, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2): 89-94.
- Mundim GJ, Paschoini MC, Júnior EA, Costa FD, Júnior VR. Assessment of angiogenesis modulators in pregnant women with pre-eclampsia: a case–control study. Arch Obstet Gynaecol. 2016;293(2):369-375.
- Shamim AA, Schulze K, Merrill RD, Kabir A, Christian P, Shaikh S, et al. First-trimester plasma tocopherols are associated with risk of miscarriage in rural Bangladesh. Am J Clin Nutr. 2015;101(2):294-301.
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
- Adekola H, Romero R, Chaemsaithong P, Korzeniewski SJ, Dong Z, Yeo L, et al. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. J Matern Fetal Neonatal Med. 2015;28(14):1621-1632.
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634-1650.
- Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3(4):228-237.
- Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort, 2. Am J Clin Nutr. 2016;103(2):454-464.
- Mol BW, Roberts CT, Thangaratinam S, Magee LA, De Groot CJ, Hofmeyr GJ. Pre-eclampsia. 2016;387(10022):999-1011.
- Oyston C, Baker PN. Therapeutic strategies for the prevention and treatment of pre-eclampsia and fetal growth restriction. Obstet Gynaecol Reprod Med. 2020;30(6): 184-189.
- Sarwar MS, Sarkar RC, Bhowmick R, Dewan SM, Ahmed MU, Hasnat A, et al. Effect of socio-economic status and estimation of lipid peroxidation and antioxidant in preeclamptic pregnant women: a case–control study. Hypertens Pregnancy. 2015;34(1):125-135.
- Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing‐related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol. 2017;77(5):1-10.
- Park S, Ham JO, Lee BK. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutr. 2015;31(1):111-118.
- Aversa R, Buzea EM, Petrescu RV, Apicella A, Neacsa M, Petrescu FI. Present a mechatronic system having able to determine the concentration of carotenoids. Am J Eng Appl Sci. 2016;9(4):1106-1111.
- Shao HB, Chu LY, Shao MA, Jaleel CA, Hong-mei M. Higher plant antioxidants and redox signaling under environmental stresses. C R Biol. 2008;331(6):433-441.
- Chen W, Maghzal GJ, Ayer A, Suarna C, Dunn LL, Stocker R. Absence of the biliverdin reductase-a gene is associated with increased endogenous oxidative stress. Free Radic Biol Med. 2018;115:156-165.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
