By Mehwish Feroz Ali1, Mervyn Hosein2, Saima Butt1, Rehan Ahmed Siddiqui3, Faraz Baig4
AFFILIATION:
- Department of Oral Pathology, Ziauddin University,.
- College of Dentistry, Ziauddin University.
- Department of Research, Ziauddin University.
- Department of Pathology, Ziauddin University, Karachi, Pakistan.
ABSTRACT
Background: Oral squamous cell carcinoma (OSCC) is the most prevalent cancer of the oral cavity representing 90% among all the common oral malignancies. It is the sixth most common cancer worldwide and ranked second highest in Pakistan. The purpose of the study was to assess the expression of zinc alpha-2 glycoprotein in oral squamous cell carcinoma and oral dysplasia. The study aimed to evaluate the association of zinc α-2 glycoprotein (ZAG) expression with different clinico-pathological parameters of OSCC.
Methods: This was a cross-sectional study conducted at Ziauddin University from January 2020-July 2020. The consecutive sampling technique was applied. In this study, 120 cases were of oral squamous cell carcinoma and 24 of oral dysplasia. The tissue samples of both cases were collected from the Histopathology Department of Ziauddin University. The zinc alpha-2 glycoprotein expression was explored through immunohistochemical analysis. The data was analyzed through SPSS and p<0.05 was considered as statistically significant.
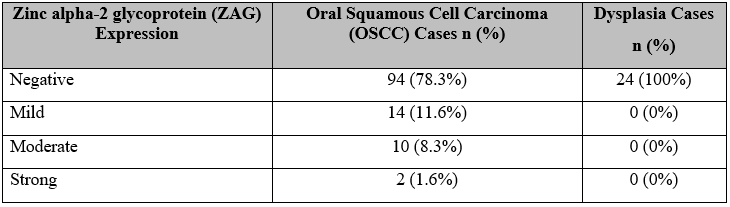
Results: ZAG positive stained OSCC cases in which 2 (1.6%) were of strong staining intensity, 10 (8.3%) moderate, and 14 (11.6%) mild, but the remaining 94 (78.3%) samples and entire oral dysplasia samples did not stain. The positive stained OSCC samples 23(46.9%) belonged to the early stages of OSCC with statistically significant results (p<0.001). All the positive stained samples were of moderately differentiated OSCC (p=0.008).
Conclusion: In this study, positive expression of zinc α-2 glycoprotein was found in the early stages (I and II) and showed a higher association with moderately differentiated tumors.
Keywords: Immunohistochemistry; Mouth Neoplasms; Precancerous Conditions; Zn-Alpha-2-Glycoprotein (ZAG).
Oral squamous cell carcinoma (OSCC) is the widest spread cancer of the oral cavity representing 90% of all the oral malignancies1. It is the sixth most common cancer worldwide and ranked the second highest in the Indian subcontinent including Pakistan2. Multiple factors contribute to the initiation and growth of OSCC in the oral epithelial cells such as smoked or smokeless tobacco, alcohol, infective agents, deficiency of nutrients, oral factors, and genetics3,4.
In Pakistan, approximately19.1% of adults and 10.7% of youth currently use tobacco in various forms1,3. The disease is prevalent in males as compared to females, a ratio of 3:1 or 3:2 respectively5. According to the literature, the most commonly affected intraoral site is the buccal mucosa in our population but in western countries, it is the tongue and floor of the mouth6. Most of the OSCC cases are reported in the middle or older age group5,7. Nowadays, the incidence of OSCC has increased in the younger age group and is constantly rising throughout the globe8.
The oral squamous cell carcinoma initially begins as a reactive or premalignant lesion over time with constant use of tobacco and products, it may transform into malignancy9. According to WHO, an oral premalignant lesion is any pathological lesion of the oral tissue that has the highest probability for malignant transformation10. It is essential to diagnose OSCC in its development phase to get good treatment outcomes and a better prognosis7.
In this study, a new biomarker, zinc alpha-2 glycoprotein (ZAG/AZGP1) expression has been investigated in the OSCC and oral dysplasia. It is an adipokine and a soluble macroglobulin with 40-kDa single-chain polypeptide10. It is synthesized mainly by the human cells like adipocytes, hepatocytes and cancerous cells11. It is also found in human fluids such as plasma, saliva, semen; CSF etc. in various metabolic and cancerous conditions11. According to the literature, in various cancerous tissues ZAG may possess immune activity against tumor antigen and prevent tumor invasion through proteolytic actions12.
Globally, very few researches have been published to assess the expression of ZAG in OSCC and other head and neck tumors13-15. To the best of our knowledge, this is the first original study conducted in Pakistan to assess the expression of ZAG in the biopsy tissue of the oral dysplasia and OSCC cases through immunohistochemistry (IHC). The study also aimed to evaluate the association of zinc α-2 glycoprotein expression in the different grades of OSCC with different clinico-pathological parameters of OSCC.
This was a cross-sectional study and the consecutive sampling technique was used. The sample size calculated from the Open-Epi software on 10% incidence, 95% confidence interval and 5% margin of error was 144, which included n=120 OSCC and n=24 Oral Dysplasia cases. OSCC and oral dysplasia paraffin-embedded tissue samples were collected from the Histopathology Department of Ziauddin University, North Campus-Karachi from January 2020 to July 2020.
In this study, the surgically resected plus diagnosed cases of OSCC that did not receive any prior chemotherapy or radiation therapy were included. The patient’s age ranged from 18-70 years and both genders were included. The recurrent OSCC, malignancy other than OSCC, improperly sectioned and stained histological slides were not considered. Ethical approval was taken from the Ethics Review Committee (ERC) of Ziauddin University, Karachi (Reference code: 1531010MFOM).
The expression of Zincs α-2 glycoprotein was investigated by immunohistochemistry using biotinylated antibodies against ZAG antigen in the tissue sample (Invitrogen, Catalog# PA5-13580). The IHC slides were interpreted by an immune-reactivity score system15, 16. The tissue sections were considered positive by dark brown cytoplasmic staining in the tumor cells observed by light microscopy. The ratio of ZAG-positive cells was counted as: 0= 5%, 1= 5%-25%, 2= 25%-50%, 3= 50%-75% and 4 =>75% respectively. Furthermore, the staining intensity of ZAG-positivity was scored as 0 = negative; 1+ = weak; 2+ = moderate; and 3+ = strong16.
Statistical analysis was performed by SPSS version 20.0. The frequency and percentage were calculated for the categorical data with mean and SD for the quantitative data. Pearson chi-square test or likelihood ratio test was applied to find the association between OSCC grading and clinical staging, staining intensity and grading, staining intensity, and clinical staging. The correlation of histological grade of oral dysplasia with gender, age, and site of the lesion was also analyzed and p-value <0.05 was considered statistically significant.
In this study, out of 120 OSCC cases, 105 (87.5%) were males and 15 (12.5%) females. Among 24 oral dysplasia cases, 16 (66.7%) were males and 8 (33.3%) females. The majority of OSCC cases occurred in those under the third 36 (30%) and fourth 33(27.5%) decade of life. In contrast, the oral dysplasia cases were predominantly in those in their fifth decade 15(62.5%). The most commonly found site of OSCC was buccal mucosa 80(67.5%) followed by the tongue 21(17.5%) and alveolar mucosa 9(7.5%). The mean age of OSCC patients was 47.2±11.06, mean tumor size 3.7±1.9, and thickness of the tumor 1.59 ± 1.26.
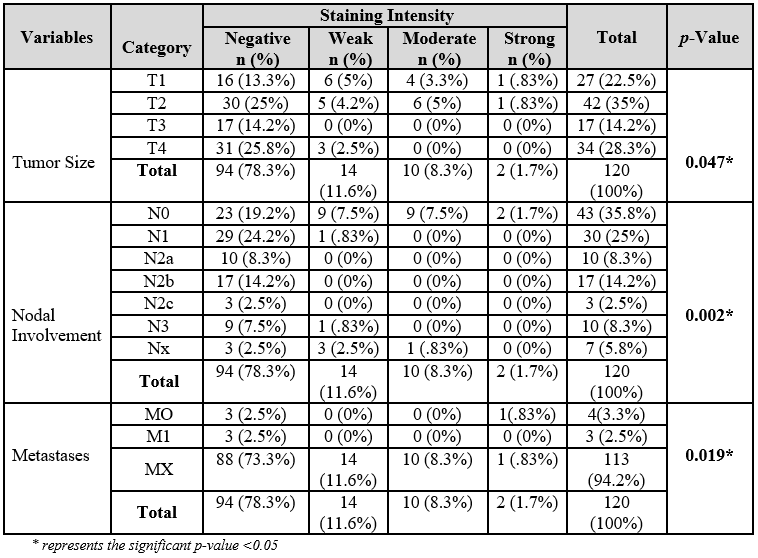
Figure 1A, 1B and1C represent the Hematoxylin and Eosin (H&E)-stained histological images of well-differentiated, moderately differentiated, and poorly differentiated OSCC respectively. Among oral dysplasia cases, there were 21(87.5%) cases of mild dysplasia, 2 (8.3%) of moderate dysplasia, and 1 (4.2%) of severe dysplasia. Buccal mucosa 18 (75%) was found to be the most common site followed by the tongue 5(21%). Table 1 represents ZAG positive stained OSCC cases in which 2 (1.6%) samples were of strong staining intensity, 10 (8.3%) moderate, and 14 (11.6%) mild, but the remaining 94 (78.3%) OSCC samples did not take up the stain. Oral dysplasia cases were negative for zinc alpha-2 glycoprotein on IHC.
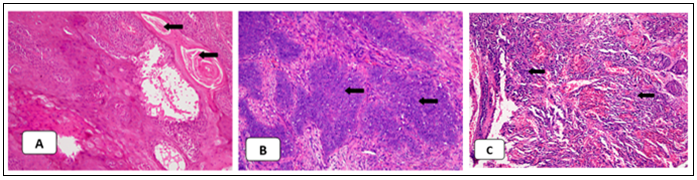
Figure 1: Hematoxylin and Eosin staining (H&E), (100 X) showing the degree of differentiation of oral squamous cell carcinoma. Arrows in image A represents keratin pearls in the well-differentiated Oral Squamous Cell Carcinoma (OSCC), image B represents moderately differentiated cancer cells, and image C shows marked pleomorphism in the poorly differentiated OSCC.
Table 1: Zinc alpha-2 glycoprotein (ZAG) expression in all cases.
The positive stained OSCC samples 23 (46.9%) belonged to early stages (stage I and II) of OSCC that show statistically significant results (p<0.001) (Table 2). All the ZAG-positive stained samples were of moderately differentiated OSCC and represented a significant association with the staining intensity (p=0.008) presented in Table 2. Hematoxylin and Eosin (H&E) and Immunohistochemistry (IHC) (Figure 2), stained histological images of OSCC in which staining intensity represented as mild intensity (1+) A, moderate (2+) C, and severe (3+) E respectively, and on other hand images B, D and F are the H&E counterparts. Table 3 displayed majority of ZAG positive samples belonged to tumor size T1 and T2 (p=0.047) and no lymph node involvement (p=0.002). The staining intensity showed positive association with those OSCC sample in which metastasis was not reported (p=0.019) (Table 3).
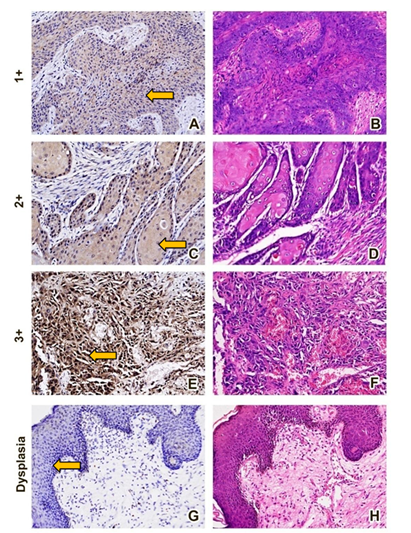
Figure 2: The arrow in image A represents the mild staining, image C moderate staining, and image E strong staining of zinc α-2 glycoprotein (ZAG) in the Oral squamous cell carcinoma (OSCC) tissue samples. The image G shows negative staining of ZAG in the oral dysplasia tissue through immunohistochemistry. Similarly, images B, D, F and H are the Hematoxylin and Eosin (H&E) counterparts of the respective Immunohistochemistry slides.
Table 2: Association of Zincs α-2 glycoprotein staining with staging and grading of oral squamous cell carcinoma (OSCC).
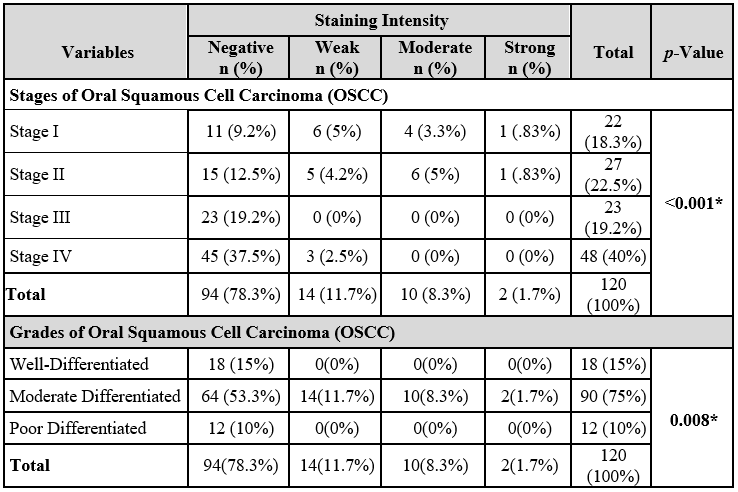
Table 3: Association of staining intensity and primary tumor: lymph nodes: metastases (TNM) status.
In this study, the oral squamous cell carcinoma samples that showed positive staining for Zinc Alpha-2 Glycoprotein (ZAG) belonged to the early stages (I and II) and of a moderately differentiated variant. This is in accordance with Poropatich et al. study; they have also found high expression of ZAG in the early stages and moderately differentiated oropharyngeal squamous cell carcinoma17. Similarly, other studies have also reported higher expression of ZAG in well-differentiated oral squamous cell carcinoma and reduced in poorly differentiated tumors18-20. This shows that the expression of ZAG was decreased with the increased rate of tumor progression21.
Similarly, Heawchaiyaphum et al. have reported increased levels of ZAG in the saliva of early-stage oral squamous cell carcinoma (OSCC) patients as compared to controls22. In this study, the association between OSCC staging and staining intensity was shown to be significant (p-value <0.001) which also indicates the positive staining in the early stages of OSCC. This is in accordance with Tang et al., which showed decreased expression of ZAG in the advanced stages of esophageal squamous cell carcinoma (ESCC) biopsy samples through immunohistochemistry (IHC) 21. In our study, the majority of positive stained OSCC samples belonged to T1 and T2 categories, no nodal involvement or N1 cases only showed positive expression. This suggests that ZAG may assist a clinician in early diagnosis and treatment planning of oral cancer as per the patient’s need and gets a tumor-free surgical margin.
There is a predominance of OSCC cases among the Pakistani males (87.5%) as compared to females (12.5%). This statistic is in accordance with the findings of several studies that have been conducted locally6,23,24. ZAG expression was positive in the majority of OSCC samples that belong to males with statistically significant results (p=0.009). This indicates that the frequent consumption of tobacco is prevalent in the male gender8,25. Recently, several studies have highlighted the occurrence of OSCC in young adults25,26. In this study, OSCC were most commonly found between the third (30%) and fourth (27.5%) decades of life. The ZAG was positively expressed in the OSCC samples belonging to this age group (p=0.016). In our population, the habit of consuming tobacco initiates from childhood, which manifests as a developed disease in adult life6.
Oral squamous cell carcinoma (OSCC) initiates as a premalignant lesion/condition and may transform into the malignancy progressively 8,9. In this study, we have assessed the expression of ZAG in those pre-malignant cases, which have been represented as dysplastic histologically; none of the oral dysplasia samples was positive for ZAG staining. This finding may suggest that ZAG biomarker can be secreted specifically by the cancer cells22. The most common site of oral dysplasia was buccal mucosa (75%) followed by the lateral border of the tongue (20.8%). Similarly, Hsue et al. had the majority of oral dysplastic lesions on buccal mucosa and tongue27. According to Singh et al., the prevalence of oral dysplasia was found in the third to fifth decade28. This is in accordance with our study, all the cases of oral dysplasia reported in the third to fifth decades. There was a significant correlation of gender (p-value=0.024) and site of lesion (p-value=0.023) with the histological grading of oral dysplasia.
In this study, 58.3% of patients are diagnosed with lymph node involvement. Study conducted by Abidi et al. found 63% of patients positive for lymph node involvement29. We observed a significant association of staining intensity with tumor size (T1 and T2) p=0.047, lymph node involvement (N0) p=0.002, and histological grading (p=0.008) but there was no association with lymphovascular invasion (p=0.812), perineural invasion (p=0.367), extracapsular spread (p=0.286) and bone invasion (p=0.438).
The above results also suggest that ZAG may be used as a potential biomarker of early diagnosis and monitoring of OSCC. Zinc alpha-2 glycoprotein may hold anti-cancer effect against the tumor cells as its high expression down-regulates the cyclin dependent kinase-2 enzymatic activities in the cell cycle15,20. Xu et al. mentioned that knockdown of ZAG would promote epithelial-mesenchymal transition, and tumor invasion by TGFβ1-ERK2 signaling pathway 30.
Studies conducted by further studies are required to find ZAG’s role as a tumor suppressor as well as a therapeutic target for OSCC and other tumors. The limitation of the study is that we cannot generalize the findings because samples were solely collected from a single institute. We have supposed that it may assist clinicians to diagnose the OSCC cases at the early stage and plan the treatment modalities as per the patient’s need. It might help surgeons to obtain a tumor-free margin, which may reduce the chances of recurrence and improve patient survival.
The positive staining of Zincs α-2 glycoprotein (ZAG) was found in the early stages and a moderately differentiated variant of Oral squamous cell carcinoma (OSCC). This may indicate that ZAG may be used as a potential biomarker for the early diagnosis of OSCC.
The authors would like to thank Dr. Adnan Zuberi, Dr, Rehan Imad and the Department of Histopathology Ziauddin Hospital North Nazimabad for their cooperation and assistance. The authors are further extending their gratitude for Dr. Afsheen Maqsood and Dr. Saeeda Baig for their valuable guidance.
The authors declare no conflict of interest.
Ethical approval was taken from the Ethics Review Committee (ERC) of Ziauddin University Karachi with the Reference code (1531010MFOM).
MF did the conceptualization of the study, literature search, data collection, analyzed the data, and written the article. MH and SB did the conceptualization, literature search, and proofreading. RA and FB did the interpretation of the results and proofreading.
- Sannam Khan R, Khurshid Z, Akhbar S, Faraz Moin S. Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: an update. Proteomes. 2016;4(4):1-11.
- Sahaf R, Naseem N, Anjum R, Nagi AH, Path FR. A study of 89 cases of oral squamous cell carcinoma presenting at Teaching Hospitals of Lahore, Pakistan. J Pak Dent Assoc. 2017;26(01):26-31.
- Asthana S, Vohra P, Labani S. Association of smokeless tobacco with oral cancer: A review of systematic reviews. Tob Prev Cessat. 2019;5:1-12.
- Salian V, Dinakar C, Shetty P, Ajila V. Etiological trends in oral squamous cell carcinoma: A retrospective institutional study. Cancer Transl Med. 2016;2(2):33-36.
- Neville BW, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. Elsevier Health Sciences; 2015. 24 p.
- Mirza D, Raza G, Basit A, Naqvi K, Ahmed S, Abassi ZA. Oral squamous cell carcinoma (OSCC) in Karachi city–a retrospective study. Pak Oral Dent J. 2016;36(3):391-394.
- Memon IM, Iqbal SM, Hussain SI, Baig MN. Pattern of oral malignancies at tertiary care hospitals. Pak J Surg. 2014;30(3):268-271.
- Mohiuddin S, Fatima N, Hosein S, Hosein M. High risk of malignant transformation of oral submucous fibrosis in Pakistani females: A potential national disaster. J Pak Med Assoc. 2016;66(11):1362-1366.
- Warnakulasuriya S, Ariyawardana A. Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med. 2016;45(3):155-166.
- Robinson M, Hunter K, editors. Soames’& Southam’s Oral Pathology. Oxford University Press; 2018. 3 p.
- Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F. Zinc α2-glycoprotein: a multidisciplinary protein. Mol Cancer Res. 2008;6(6):892-906.
- Tsai JS, Chen SC, Huang KC, Lue BH, Lee LT, Chiu TY, et al. Plasma zinc α2‐glycoprotein levels are elevated in smokers and correlated with metabolic syndrome. Eur J Clin Invest. 2015;45(5):452-459.
- Vidotto A, Henrique T, Raposo LS, Maniglia JV, Tajara EH. Salivary and serum proteomics in head and neck carcinomas: before and after surgery and radiotherapy. Cancer Biomark. 2011;8(2):95-107.
- Chen Y, Azman SN, Kerishnan JP, Zain RB, Chen YN, Wong YL, et al. Identification of host-immune response protein candidates in the sera of human oral squamous cell carcinoma patients. PLoS One. 2014;9(10):1-10.
- Brysk MM, Lei G, Adler-Storthz K, Chen Z, Brysk H, Tyring SK, et al. Zinc-α2-glycoprotein expression as a marker of differentiation in human oral tumors. Cancer Lett. 1999;137(1):117-120.
- Ali MF, Hosein M, Butt S, Siddiqui RA. Zinc α-2 glycoprotein (ZAG) a novel biomarker for the detection of oral squamous cell carcinoma. Pak J Med Dentist. 2020;9(3):77-83.
- Poropatich K, Paunesku T, Zander A, Wray B, Schipma M, Dalal P, et al. elemental Zn and its Binding protein Zinc-α2-Glycoprotein are elevated in HpV-positive oropharyngeal squamous cell carcinoma. Sci Rep. 2019;9(1):1-1.
- Xue Y, Yu F, Yan D, Cui F, Tang H, Wang X, et al. Zinc-α-2-glycoprotein: a candidate biomarker for colon cancer diagnosis in Chinese population. Int J Mol Sci. 2015;16(1):691-703.
- Zhao J, Fan YX, Yang Y, Liu DL, Wu K, Wen FB, et al. Identification of potential plasma biomarkers for esophageal squamous cell carcinoma by a proteomic method. Int J Clin Exp Pathol. 2015;8(2):1535-1544.
- Lei G, Brysk H, Arany I, Tyring SK, Srinivasan G, Brysk MM. Characterization of zinc‐α2‐glycoprotein as a cell adhesion molecule that inhibits the proliferation of an oral tumor cell line. J Cell Biochem. 1999;75(1):160-169.
- Tang H, Wu Y, Qin Y, Wang H, Wang L, Guan X, et al. Reduction of AZGP1 predicts poor prognosis in esophageal squamous cell carcinoma patients in Northern China. Onco Targets Ther. 2017;10: 85-94.
- Heawchaiyaphum C, Pientong C, Phusingha P, Vatanasapt P, Promthet S, Daduang J, et al. Peroxiredoxin-2 and zinc-alpha-2-glycoprotein as potentially combined novel salivary biomarkers for early detection of oral squamous cell carcinoma using proteomic approaches. J Proteom. 2018;173:52-61.
- Khaleel ME, Raza A, Ehsan A, Masood R, Javed M. Clinicopathological spectrum of oral squamous cell carcinoma at a public sector health facility. Biomed. 2015;31(1):21-26.
- Gupta N, Gupta R, Acharya AK, Patthi B, Goud V, Reddy S, et al. Changing Trends in oral cancer-a global scenario. Nepal J Epidemiol. 2016;6(4):613-619.
- Akram S, Mirza T, Mirza MA, Qureshi M. Emerging patterns in clinico-pathological spectrum of oral cancers. Pak J Med Sci. 2013;29(3):783-787.
- Ahmed R, Malik S, Khan MF, Khattak MR. Epidemiological and clinical correlates of oral squamous cell carcinoma in patients from north-west Pakistan. J Pak Med Assoc. 2019;69(8):1074-1078.
- Hsue SS, Wang WC, Chen CH, Lin CC, Chen YK, Lin LM. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: a follow‐up study based in a Taiwanese hospital. J Oral Pathol Med. 2007;36(1):25-29.
- Singh S, Singh J, Chandra S, Samadi FM. Prevalence of oral cancer and oral epithelial dysplasia among North Indian population: A retrospective institutional study. J Oral Maxillofac Pathol. 2020;24(1):87-92.
- Abidi F, Hosein M, Butt SA, Zaidi AB, Anjum A, Fatima S. Association of clinicopathological features with lymph node metastasis: A cross sectional study of oral squamous cell carcinoma patients. J Adv Med Med Res. 2020:46-53.
- Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer Lett. 2016;374(2):241-249.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
