By Humaira Ansari1, Shumaila Usman2, Syed Tousif Ahmed1, Shazia Hashmat1
AFFLIATIONS:
- Department of Physiology, Ziauddin University, Karachi, Pakistan.
- Department of Research, Ziauddin University, Karachi, Pakistan.
ABSTRACT
The neurodegenerative disorder is a prolonged persistence curse and effect on economic and physical challenges in an aging world. Parkinson has come in the second category of disability disorders and associated with progressive dopaminergic neuronal degeneration with severe motor complications. It is an observation that gradual disease progression causes 70% degeneration of striatal dopaminergic neurons. Globally there are around 7-10 million patients with Parkinson’s disease, however, there are huge efforts for therapeutic improvement. According to studies, no single molecular pathway was pointed out as a single etiology to control disease progression due to a lack of targeted therapeutic strategies. Previously implemented symptomatic treatments include L-dopa (L-3,4-dihydroxyphenylalanine), deep brain stimulation, and the surgical insertion of a medical device. This leads to dyskinesia, dystonia and a higher risk of major surgical complications respectively. However, not all the above-mentioned therapies cannot regenerate the dopaminergic neurons in Parkinson’s disease patients. Recent advances in the field of cellular therapy have shown promising outcomes by differentiation of multipotent mesenchymal stem cells into dopaminergic neurons under the influence of a regenerative substance. In this review, we have discussed the differentiation of dopaminergic neurons by using different cell types that can be used as a cellular therapeutic approach for Parkinson’s disease. The information was collected through a comprehensive search using the keywords, “Parkinson Disease, Dopamine, Brain derived neurotrophic factor and neuron from reliable search engines, PubMed, Google Scholar and Medline reviews from the year 2010 to 2020.
Keywords: Parkinson Disease; Dopamine; Brain Derived Neurotrophic Factor; Neuron.
Parkinson’s disease (PD) is a neurodegenerative progressive disorder affecting millions of people worldwide. It is characterized by typical movement disorder including rigidity, tremors and bradykinesia1. It is identified pathogenically, the Lewy bodies made up of misfolded forms of the protein α-syn2.
Pharmacological treatment can be advantageous for many years but prolong use of pharmacological treatment had produced many side effects including, on-off fluctuations and wearing-off phenomenon3, confusion, hypotension, hallucination, orthostatic hypotension, fatigue, brain hemorrhage, infarction, and seizures4.
Other innovative modalities had been introduced to PD consisting of gene therapy and cellular therapy. Gene therapy produces enzymes which are responsible for non-synaptically, generating L-dopa (L-3,4-dihydroxyphenylalanine) and dopamine (DA) but this treatment had not proved to be fruitful because this therapy works without any feedback regulation of cellular specificity5.
Another new modality is cell-based therapy that has attracted the attention of researchers as being the potentially feasible novel therapies for neurodegenerative diseases. It encompassed the derivation of specific neuronal subtypes lost in the disease and consequent transplantation into exaggerated areas of the nervous system cell types had been used for the differentiation of dopaminergic neurons for the subsequent transplantation into the Parkinson’s patient6. In this review, we have discussed the different strategies and cell types used for the differentiation of dopaminergic neurons.
Cellular therapy is considered as the elementary unit of regenerative medicine7 in the upcoming fields. The goal is to restore the lost function rather than produce a new organ. The differentiated mesenchymal stem cells (MSCs) are plentifully used as a cellular therapy for degenerative diseases. Besides MSCs, other stem cells have also varying differential potential and can be obtained from different sources9.
Embryonic Stem Cells (ESCs)
Embryonic stem cells (ESCs) are derived by the blastocyst. The major characteristics have a high capability for potency and self-renewal. ESCs are formed into three germ layers endoderm, mesoderm and ectoderm and the formation of the specific organs. Some progenitor cells retained the organ, can proliferate under injury repair, and can differentiate. These tissue stem cells are found in bone, blood, muscle, adipose tissue, liver, brain, skin, gastrointestinal tract and bone marrow. These cells have the potential to differentiate into three three primary germ layers and also sustain in an undifferentiated state and also survive in culture media for a long time.10
The following transcriptional factors of ESCs e.g., Nanog and Oct4 maintain an undifferentiated state of the stem cells and self-regeneration ability. It was observed that no genetic aberrations are proliferated in the ESC line. Embryonic stem cells are cultured in a media containing the anti-differentiation cytokine in leukemia inhibitory factors (LIF). Withdrawal of the embryonic stem cells from the feeder film consequently caused the development of “embryoid bodies”, having all three germs layer10 however; their use is restricted due to ethical issues and high risk of teratoma formation after transplantation10.
Induced Pluripotent Stem Cells (IPSCs)
Adult somatic stem cells can convert induced pluripotent stem cells because these cells are genetically reprogrammed and convert into embryonic stem cells (ESC). Now iPSCs are a very important tool for drug development, regenerative medicine and modeling diseases. Further research also showed other numerous benefits, a cell source for Parkinson’s disease (PD) replacement as well as the capability to use patient’s peculiar cells and consequently reduced the necessity for immuno-suppression. Furthermore, a clinical trial was initiated in Japan, Takahashi’s lab, where PD patients have received human leukocyte antigen (HLA) matched iPSCs derived dopamine neurons. All these researches can be used as assistance for future approach3; however, it has limitations due to the high risk of teratoma formation after transplantation9.
Differentiation of IPSCS
Bone marrow mesenchymal stem cells (BMSCs) have great potential to differentiate into neuron-like cell by the presence of the following factor such as hepatocyte growth factors (HGF), vascular endothelial growth factors (VEGF), epidermal growth factors (EGF). These differentiated cells were identified through immunocytochemistry; phase contrast inverted microscopy, immunocytochemistry and transcriptase-polymerase chain reactions to identify the neuronal specific markers11.
Sympathetic neurons can also be derived from induced pluripotent stem cells and embryonic stem cells. Scientists used the helix-loop-helix, Achaete-scute homolog 1 (ASCL1), Paired-like homeobox (PHOX2B), homeodomain transcription factor as important factors for sympathetic neuronal development. However, they also showed the activation of Wingless-related integration site (WNT) signaling pathway12. Neural differentiation derived from iPSCs by the induction of brain derived growth factors and neuronal cells production with better dendritic, axonal growth and intracellular connectivity can be visible within seven days13.
According to Cheng et al. rat bone marrow mesenchymal stem cells differentiate into dopamine neuron like cells by the induction of liver X receptor, causes well differentiation of BMSCs into dopamine neurons14. This research also showed that when carbonized substrate cultured on human neuronal stem cells, they could be differentiated into matured and specialized neuron cells. Literature also showed that carbon material like graphene oxide promotes the mouse embryonic stem cells differentiation into the dopaminergic neuron, with neuronal gene expression markers15. Another scientist Velasco et al differentiated hESCs and iPSCs into mature neurons under the influence of transcription factor either NeuroD1 or Neurogenin 2 within two weeks16.
Adult Stem Cells
Application of adult stem cell therapy is better compared to the utilization of aborted human embryo’s ventral mDA17 because of immune reactions causes dysfunction of transplanted DA neurons18. Adult stem cells are obtained from all tissues of the three germ layers and also from the placenta for example, MSCs can be derived from human amnion epithelial cells. These stem cells had various properties such as anti-inflammatory, no rejection issue, and limited differentiation ability. In vitro they are differentiated into different germ cell layers. Numerous researches had proved in vivo transplantation of adult stem cell repairs of the injured organs, like bone tissue repair and new specialized cell generation. Revascularization of the ischemic cardiac tissue also proved that adult stem cells could release many molecular mediators, with immunomodulatory, angiogenic and anti-apoptotic chemoattractant properties, that stimulate the healing process in culture media9. The usage of hMSCs in cell based therapy had involved wide interest in the applications of numerous incurable diseases and had revealed several superior characteristics for therapeutic usage compared to other types of stem cells which are discussed below.
Mesenchymal Stem Cell (MSCs)
Mesenchymal stem cells have prodigious potential with a big source for cellular therapy for neuronal disorders. MSCs are the best example of adult multipotent progenitor cells, derivative from several adult tissues and in vitro they have the ability of self-renewal. The discovery of MSCs from both human and mouse origins can be differentiated into functional neurons and encouraged as a replacement of impaired neurons19.
The mesenchymal stem cells are spindle-shaped morphologically. They can adhere to tissue culture plastic and grow in culture media and can expand and maintain the multipotent characteristics19. They are easy to separate, more multilineal differentiation integration into a strong functional shape20 and are unrestricted from ethical problems but they have restricted replicative life span9. MSCs release the soluble factors that are significant for cell existence and proliferation, control immune responses and migration to the particular site of injury21.
MSCs are obtained from many tissues: adipose tissues, bone, bone marrow, peripheral blood, Wharton’s jelly, dental pulp, decidua basalis, amniotic fluid, umbilical cord, placenta, and amniotic membrane, chorionic villi from human placenta, menstrual blood, breast milk, and urine etc22. MSCs with their novel therapeutic properties are the most significant cell type in the era of tissue engineering and cell based therapy23. Besides their wide availability of proliferative capacity and multipotency, they are the most clinically practiced cell source24. They aid in the release of neurotrophic and angiogenic factors that stimulate neuronal growth-enhancing synaptic connections, promote angiogenesis, neurogenesis, differentiation and axonal remyelination25.
MSCs is the best source for cell-based therapy hence it is safely used for autologous transplantation and showed high differentiation potential26, no tumorigenicity due to paracrine secretion of these cells, their property exhibits wide clinical potential by regulating apoptosis, angiogenesis, cell differentiation, immunomodulation and extracellular matrix composition19. The importance of mesenchymal cells, their differentiation ability, potential regeneration of the desire cells, all these unique properties are discussed in this review.
Mesenchymal Stem Cells Based Therapies for Neurologlogical Diseases
Mesenchymal Stem Cells (MSCs) have unique features as compare to other stem cells. These cells have been used in different clinical trials including, Parkinson’s disease, Spinal cord injury, Huntington’s disease, multiple sclerosis and brain ischemia27.
The human mesenchymal stem cells (hUMSCs) is a high source of progenitor and stem cells containing mesenchymal stem cells. hUMSCs are achieved either from cord blood or the cord tissue. hUMSCs have numerous benefits as compared to other sources of stem cells. MSCs show (Figure 1) various cell surface antigens including CD 105, CD 73, CD90, CD146, and various integrins and adhesion molecules. They can easily be harvested with no harm to the baby and mother. They exhibit high proliferative property and low immunogenicity28. Due to the abundant availability of the umbilical cord, its compatible and effective clinical application can be considered as one of the most plentiful sources of non-embryonic stem cell29.
Differentiation of HUMSCS into Dopaminergic Neurons like Cells
Differentiation of dopaminergic neurons can be achieved by the exposure of extrinsic factors30, including Wnt family members, soluble proteins, growth factors (FGF, GDNF, BDNF, TGFβ2 and EGFR) and chemical inducers (dbcAMP, AA and BHA)31. When hUMSCs were cultured in neural containing medium, they produced neural morphologies and expressed neural markers such as nestin, NeuN and glial fibrillary acidic protein27. The Wharton’s jelly of the MSCs transdifferentiated into neuron-like cells and they expressed Nestin and Neuro-D1 as neuronal markers by using Valproic acid32.
Previously MSCs from human Wharton jelly was successfully induced by using a glial cell-derived neurotrophic factor and brain derived neurotrophic factors into neurons and auditory hair cells during in vitro experiment33. Human umbilical cord tissues differentiated into neuron like cells by using nestin, GFAP and synaptic markers: SYN, PSD95, and GAP4334. Mallis et al.35observed WJ-MSCs differentiated into neural-like cells by using basic fibroblast growth factor and Forskolin, thus differentiating cells into neuron-like cells and astrocytes by cAMP-elevating agents such as Forskolin and 3-isobutyl-1-methylxanthine, and also without the presence of growth factors36.
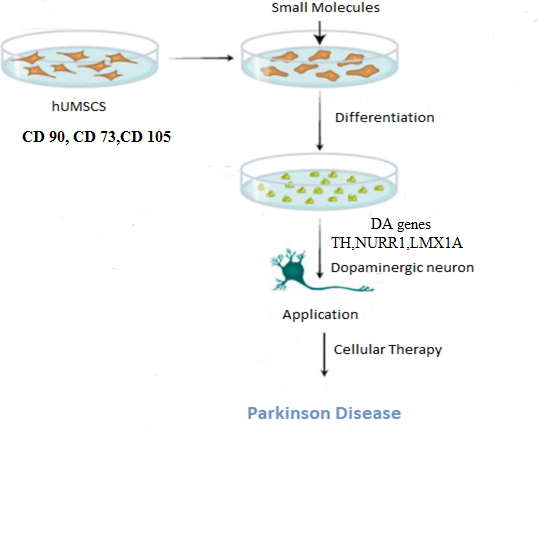
Figure 1: Human umbilical cord mesenchymal cells (huMSCs) differentiation into dopaminergic neurons.
Dopaminergic Neuron Differentiation from Other Sources
Many sources were identified from which dopaminergic neuron differentiation from stem cells was initiated for future applications in regenerative medicine. One of the promising sources is Human olfactory ectomesenchymal stem cells (OE-MSCs) which can differentiate into dopaminergic (DA) neurons by maintaining their plasticity. OE-MSCs were potentiated to differentiate into DA neuron-like cells in vitro by the induction of sonic hedgehog (SHH) signaling pathway, basic fibroblast growth factor (bFGF), fibroblast growth factor 8, Glial cell line-derived neurotrophic factor (GDNF) and brain derived neurotrophic factor (BDNF)29. Human dental pulp stem cell (hDPSCs) differentiation to dopaminergic neurons has been proved a high source. The hDPSCs are obtained noninvasively by deciduous teeth and sustain their multipotency with neuron-like cells and self-renewal properties. Knockout-embryonic stem cell (KO-ES) medium with leukemia inhibitory factor (LIF) was used in which the human pulp cells were expanded. Within 4 days, the neurosphere was formed and further transferred into ITS (human insulin transferrin sodium) and fibronectin media, which further confirmed the selection for Nestin-positive cells. In conclusion, the cells were moved into N-2/ascorbic acid media to promote dopaminergic neurons by the differentiation process and observing the expressions of mesenchymal stem cell markers and early neuronal markers at different stages37.
According to Lairson et al, fluoxetine stimulates neurogenesis in neural stem cells within the hippocampus. This result showed that there is a link between the regeneration of neurons and their role in reducing the symptoms of depression38. Another research showed that MSCs can be differentiated into osteogenic cells and expressed the bone markers under the induction of osteogenic differentiation medium. In the stage of proliferation, MSCs release laminin, fibronectin and collagen type I in the matrix maturation stage. They secrete alkaline phosphate and during matrix, mineralization expressed the bone genes such as osteopontin proteins and sialoprotein39.
It was also observed that statin molecules are effective in both neuronal differentiation and midbrain neuron specification. Other statin molecules are less potent in neuronal differentiation as compared to mevastatin such as simvastatin40. Neural progenitor cells upon differentiation into dopaminergic neurons by mevastatin had shown high expression of dopamine specific genes including TH, Nurr1 and LMX1a compared to other statin molecules. Statin had shown noteworthy effects in both promoting the midbrain neuron specification and neuronal lineage differentiation. Moreover, its efficient mechanism of apoptosis induction in the undifferentiated cells eliminates the chances of brain tumor cancer stem cells41. Gonzalez et al. reported that neural stem cell differentiation into dopamine neurons in the presence of guggulsterone would be a new approach for the treatment of Parkinson’s disease42. According to Osborn in the area of transplantation, immune compatibility is very significant and these strategies have fewer advantages to the patients rather than an autologous approach.
Advantages of Autologous Transplantation for Parkinson’s Disease
- In Parkinson’s patients, no immune suppression by autologous transplantation.
- These are serious immunological reactions of allogeneic transplantation.
- Neural cell autologous approach potentially is greater incorporated the axonal functional3.
- This therapy is effective for neurodegenerative diseases such as Parkinson diseases as shown in Table 1.
Table 1: Overview of advantages and disadvantages of different types of stem cell for cellular therapy of Parkinson’s disease43.
| Stem Cells | Advantages | Disadvantages |
| ESCs | High proliferative capacity | High risk of teratoma formation |
| High potential to differentiate ant cell types | High risk of immune rejection | |
| Ethical issues | ||
| NSCs | Limited differentiation potential | Restricted proliferative capacity |
| Decrease the risk of tumor formation | Low neuronal differentiation capacity | |
| Ethical issues | ||
| Risk of immune rejection | ||
| MSCs | High proliferative capacity | Differentiated into DA |
| No ethical issues | ||
| iPSCs | High proliferative capacity | Risk of teratoma formation |
| No ethical issues | Differentiation of DA | |
| No immune rejection |
Therapeutic strategies for neurodegenerative disorders have been found mandatory requirement and have proven to be a major promising achievement for neurological diseases like Parkinson’s disease. Damage to the dopaminergic neurons in the Substantia Nigra being the sole cause of movement disorders in PD patients has led to many innovative therapies like drug therapies acting on receptors but then wearing off side effects prevail. Deep brain stimulation also facing different limitations in the regeneration of dopaminergic neurons has led tone modalities to be investigated gradually. Emerging options of dopaminergic neuron regeneration by Human Mesenchymal Stem Cells differentiation has been proved the best source of cell-based therapies for neurological diseases. The source of MSC has been exhibited through other sources like Human Dental Pulp cells, Human Olfactory Ectomesenchymal stem cells and hUMSCs, statin through gene expression of dopamine. These examples are a high source of dopaminergic neuron differentiation, which were initiated for future application in regenerative medicine.
I would like to thank Dr. Nabila for her valuable support towards experimental work support.
There was no conflict of interest among the authors.
HA and SU performed the conceptualization of the study, literature search and prepared the manuscript. ST did the overall evaluation of the review and SH helped in the preparation of the manuscript.
- Engelender S, Isacson O. The threshold theory for Parkinson’s disease. Trends Neurosci. 2017;40(1):4-14.
- Moutinho M, Codocedo JF, Puntambekar SS, Landreth GE. Nuclear receptors as therapeutic targets for neurodegenerative diseases: lost in translation. Annu Rev Pharmacol Toxicol. 2019;59:237-261.
- Osborn TM, Hallett PJ, Schumacher JM, Isacson O. Advantages and recent developments of autologous cell therapy for Parkinson’s disease patients. Front Cell Neurosci. 2020;14:1-13.
- Wang Y, Ji X, Leak RK, Chen F, Cao G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res Rev. 2017;34:39-50.
- Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115(1):19-38.
- Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative Diseases. Ann Neurol. 2011;70(3):353-361.
- Teo AK, Vallier L. Emerging use of stem cells in regenerative medicine. Biochem J. 2010;428(1):11-23.
- Sánchez A, Schimmang T, García-Sancho J. Cell and tissue therapy in regenerative medicine. InStem Cell Transplantation: Springer, New York, NY; 2012. p. 89-102.
- Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21(1):1-10.
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3-10.
- Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52(3):401-412.
- Oh Y, Cho GS, Li Z, Hong I, Zhu R, Kim MJ, et al. Functional coupling with cardiac muscle promotes maturation of hPSC-derived sympathetic neurons. Cell Stem Cell. 2016;19(1):95-106.
- Lin MH, Chung CY, Chen KT, Yeh JC, Lee TH, Lee MH, et al. Comparison between polybutylcyanoacrylate nanoparticles with either surface-adsorbed or encapsulated brain-derived neurotrophic factor on the neural differentiation of iPSCs. Int J Mol Sci. 2019;20(1):1-23.
- Cheng O, Tian X, Luo Y, Mai S, Yang Y, Kuang S, et al. Liver X receptors agonist promotes differentiation of rat bone marrow derived mesenchymal stem cells into dopaminergic neuron-like cells. Oncotarget. 2018;9(1): 576-590.
- Ferraro RM, Ginestra PS, Giliani S, Ceretti E. Carbonization of polymer precursors substrates to direct human iPSC-derived neurons differentiation and maturation. Procedia CIRP. 2020;89:39-44.
- Velasco I, Salazar P, Giorgetti A, Ramos‐Mejía V, Castaño J, Romero‐Moya D, et al. Concise review: generation of neurons from somatic cells of healthy individuals and neurological patients through induced pluripotency or direct conversion. Stem Cells. 2014;32(11):2811-2817.
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15(5):653-665.
- Studer L. Strategies for bringing stem cell-derived dopamine neurons to the clinic—the NYSTEM trial. InProgress in brain research: Elsevier; 2017. p. 191-212.
- Volkman R, Offen D. Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells. 2017;35(8):1867-1880.
- Green EM, Lee RT. Proteins and small molecules for cellular regenerative medicine. Physiol Rev. 2013;93(1):311-325.
- Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829-848.
- Van Pham P, Truong NC, Le PT, Tran TD, Vu NB, Bui KH, et al. Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell Tissue Bank. 2016;17(2):289-302.
- Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow‐derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32(7):1713-1723.
- Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016;2016:1-16.
- Castillo-Melendez M, Yawno T, Jenkin G, Miller SL. Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Front Neurosci. 2013;7:194-207.
- Chang YH, Wu KC, Liu HW, Chu TY, Ding DC. Human umbilical cord-derived mesenchymal stem cells reduce monosodium iodoacetate-induced apoptosis in cartilage. Tzu Chi Med J. 2018;30(2):71-80.
- Stoddard-Bennett T, Reijo Pera R. Treatment of Parkinson’s disease through personalized medicine and induced pluripotent stem cells. Cells. 2019;8(1):26-40.
- Dalous J, Larghero J, Baud O. Transplantation of umbilical cord–derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res. 2012;71(2):482-490.
- Ali H, Bahbahani H. Umbilical cord blood stem cells–potential therapeutic tool for neural injuries and disorders. Acta Neurobiol Exp (Wars). 2010;70(3):316-324.
- Pan G, Liu J. Small molecules and extrinsic factors promoting differentiation of stem cells into insulin-producing cells. Ann Endocrinol. 2019; 80(2):128-133.
- Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nat. 2002;418(6893):50-56.
- Ghorbani S, Tiraihi T, Soleimani M. Differentiation of mesenchymal stem cells into neuron-like cells using composite 3D scaffold combined with valproic acid induction. J Biomater Appl. 2018;32(6):702-715.
- Kil K, Choi MY, Park KH. In vitro differentiation of human Wharton’s jelly-derived mesenchymal stem cells into auditory hair cells and neurons. J Int Adv Otol. 2016;12(1):37-42.
- Shi Y, Nan C, Yan Z, Liu L, Zhou J, Zhao Z, et al. Synaptic plasticity of human umbilical cord mesenchymal stem cell differentiating into neuron-like cells in vitro induced by edaravone. Stem Cells Int. 2018;2018:1-12.
- Mallis P, Papadopoulos G, Michalopoulos E. Wharton jelly mesenchymal stem cells derived from human umbilical cord capable to differentiate into neural-like cells and their potential use in neurological disorders. Electronic J Biol. 2017, 13(4): 442-461.
- Shahbazi A, Safa M, Alikarami F, Kargozar S, Asadi MH, Joghataei MT, et al. Rapid induction of neural differentiation in human umbilical cord matrix mesenchymal stem cells by cAMP-elevating agents. Int J Mol Cell Med. 2016;5(3): 167-177.
- Chun SY, Soker S, Jang YJ, Kwon TG, Yoo ES. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. J Korean Med Sci. 2016;31(2):171-177.
- Lairson LL, Lyssiotis CA, Zhu S, Schultz PG. Small molecule–based approaches to adult stem cell therapies. Annu Rev Pharmacol Toxicol. 2013;53:107-125.
- Antebi B, Zhang Z, Wang Y, Lu Z, Chen XD, Ling J. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng Part C Methods. 2015;21(2):171-181.
- Sucu S, Brunner-Lorieux S, Hommet Y, Binet V, Bideau E, Debroas E, et al. Statins induce both neuronal-like differentiation and programmed death in PC 12 cells [dissertation on the internet]. Universite de CAEN; 2016. [cited 2020 Sep 7]. Available from: https://www.researchgate.net/profile/Dominique_Duval/publication/307575962
- heon Rhim J, Luo X, Xu X, Gao D, Zhou T, Li F, et al. A High-content screen identifies compounds promoting the neuronal differentiation and the midbrain dopamine neuron specification of human neural progenitor cells. Sci 2015;5:1-14.
- Gonzalez R, Garitaonandia I, Abramihina T, Wambua GK, Ostrowska A, Brock M, et al. Deriving dopaminergic neurons for clinical use. A practical approach. Sci 2013;3:1-5.
- Jensen P, Krabbe C, Meyer M. Cell Therapy for parkinson’s disease: status and perspectives. Transl Neurodegener. 2011: 343-378. InTech-Open Access Publisher.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
