By Anam Shaikh, Fouzia Lateef, Talat Mirza
AFFLIATIONS:
Department of Histopathology, Ziauddin University and Hospital, Karachi, Pakistan.
ABSTRACT
Renal disease and dysfunction is a worldwide public health problem. The underlying pathology in most renal disease is glomerulopathy, largely referred to as glomerulonephritis. It can be primary or secondary to other diseases. A range of morphological patterns was observed in this condition, each with different etiopathogenetic mechanisms, diverse clinical presentation, disease progression and therapeutic responses. We searched the literature using Hinari, PubMed and Google Scholar, for appropriate studies. This review was conducted by employing specified methods and structures using histopathology-confirmed data during the year 2011 to 2020. Thirty-five studies consisting of 13,423 reported renal biopsy cases were covered in this review. The most common indication of the renal biopsy was nephrotic syndrome followed by proteinuria and nephritic syndrome. Focal segmental glomerulosclerosis, minimal change disease, and mesangio-capillary glomerulonephritis among others, were the most frequently reported primary patterns of glomerulopathies. Glomerular diseases remain poorly characterized due to the scarcity of data on histo-morphological patterns of glomerulopathies. The development of registries regarding renal biopsy may offer a chance to characterize the pervasiveness and patterns of glomerulopathies and have a positive impression on chronic/end stage renal disease analysis and treatment since most glomerular diseases are complaisant to treatment.
Keywords: Nephrotic Syndrome; Immunofluorescence; Biopsy; Glomerulonephritis.
Renal diseases are a global health issue. According to the World Health Statistics 2016 and Sustainable Development Goals (SDG) project, the kidney and urinary tract pathologies, confers to the worldwide burden of diseases, with around 850,000 deaths and 15,010,167 fine-tune conditions every year1. They are the 12th cause of death and the 17th cause of disability2.
Pakistan ranks eighth in renal disease causing 20,000 deaths every year. Additionally, among the 43,000 people dying in the country due to organ failure, more than 45% die of renal failure (both acute and chronic) 3. The underlying pathology in most cases (33.56%) of renal dysfunction is glomerular dysfunction/disease, largely referred to as glomerulonephritis (GN) 4,5.
In developing countries, glomerulonephritis is a highly reported diagnosis to patients carrying the burden of renal diseases6,7. According to the 2012 United States Renal Data System (USRDS) 28.3/million/year is the adjusted renal disease rate due to primary glomerulopathy8. Material from the Chinese Renal Data System reveals that the most common cause of renal pathology is glomerular diseases (57.4%) 9. Data from Africa displays glomerular diseases in 10.2% to 52% of patients with renal disease10,11.
A variety of morphological pictures are noted in the condition inclusive of minimal change disease, glomerular basement membrane thickening, mesangioproliferative glomerulonephritis, focal and segmental scarring, mesangiocapillary glomerulonephritis, hyalinosis and other rare patterns12. Despite the high prevalence and incidence of glomerular disease, the underlying histological variants and morphological patterns are seldom explored, resulting in a lack of understanding of the morphological patterns of the disease.
Recent studies are now showing, morphological patterns of glomerular diseases having different etiological factors, pathogenetic mechanisms and diverse clinical presentation, proteinuric remission, disease progression and therapeutic response13,14, making it even more important to investigate the matter as this may lead to better prognosis and reduced morbidity and mortality from the disease.
Nephrotic syndrome is a combination of clinical presentation and laboratory outcomes embracing non-selective proteinuria (3.5 g/24 h), low level of albumin in the blood, increased level of cholesterol, and generalized edema14-16. Nephrotic syndrome is the most frequent cause of glomerular damage. The glomerular impairment may be unspecified (primary or idiopathic) or because of some known disorders like Systemic lupus erythematosus (SLE) and Henoch- Schonlein purpura (HSP) which is a secondary glomerulonephritis17.
About 60% of the adult population developed primary GN18. There is a very little amount of fluorescence-based documentation in local data particularly, regarding the pathology of adult nephrotic syndrome cases. As per literature, very few past studies were found, purely made diagnosis on light microscopy without the use of immunofluorescence or electron microscope and thus the real picture of glomerular injury was not reflected in nephrotic syndrome19. Therefore, these types of studies missed-diagnosed as mesangiocapillary and mesangioproliferative glomerulonephritis, while most excluding other entities like focal segmental glomerulosclerosis20.
According to current reports, the various region of the world shows the root of nephrotic syndrome is focal segmental glomerulosclerosis21, followed by membranous glomerulonephritis and minimal change disease with many of other least common patterns. The cases of nephritic syndrome mostly diagnosed as IgA nephropathy22-24.
In the 1970s, the International Study of Kidney Diseases in Children reported minimal change disease is the most common histopathological pattern in biopsies from individuals with idiopathic nephrotic syndrome23. Minimal Change disease reported by other single center studies in 70-90% of cases. Focal segmental glomerulosclerosis was the underlying cause of idiopathic nephrotic syndrome found in only 5-7% of cases25. It is believed, however, that children and adults both are on the higher incident of focal segmental glomerulosclerosis26. However, there is no comprehensive review of literature reporting the morphological pattern of glomerulonephritis. Consequently, we line-up to review in stock published literature on the histomorphological spectrum of glomerular diseases.
This review was conducted according to the guidelines of “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)”. Studies were collected by using PubMed, Hinari and Google Scholar as a search engine. The search of studies was restricted to three keywords (i.e., Histo-morphology, Glomerulonephritis and Glomerulopathies using the Boolean operator “and”) and search results from last decade (2011-2020) were included. Only articles available in English were included. The bibliographies of articles were also inspecting to strengthen the search.
We included, study, which reports only biopsy-proven cases of glomerulopathies, contain a minimum of 50 participants and provided data on morphological types of the reported glomerulopathies. The study whose focus was on the comorbid condition and whose whole unit had a single or specific type of glomerulopathy was excluded. The relevant data were extracted to review the full-text study of selected data. Data collection included the region and country of publication, publication year, the design of the study, number of performed biopsies and their indication, distribution of gender, frequencies of reported histomorphological types and their etiopathogenesis.
Thirty-five complete research papers were reviewed and analyzed for this review study. Out of 35 studies, 18 (51.43%) reported on adults (1st group), 11/35 (31.43%) comprising of adults and pediatrics (2nd group) and 6/35 (20.69%) were limited to pediatric age group (3rd group) (Figure 1). Total, 13,423 documented cases of renal biopsy in the last decade (2011-2020) were evaluated. The study duration stretched from 12 months to up to many years. The prospective study design was found only in eight studies.
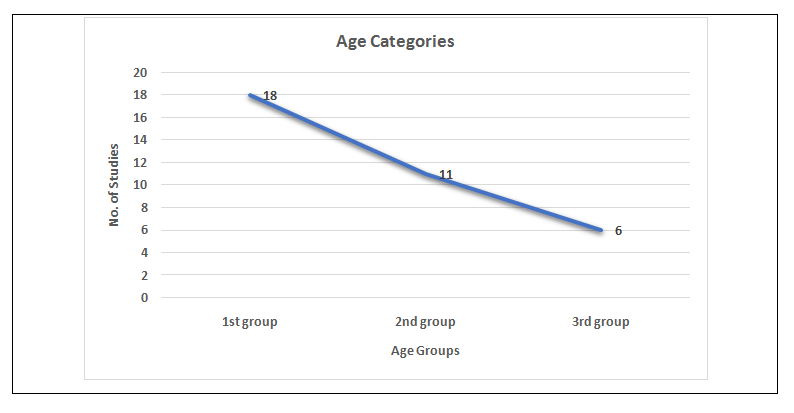
Figure 1: Data regarding the age categories of group individuals.
Male predominance observed in most of the studies: 45.2%-63.7% in 1st group; 47.3%-64.3% in 2nd group and 53.0%-68.8% in 3rd group. In 1st group studies, at the time of biopsy the average age was below 40 years (range 15-70 years), while in 3rd group studies, the average age was below 9.5 years (range 1-14 years). Generally, we found 121 was the median number of cases per the study, however, the vast difference observed in the mean rate of renal biopsy cases reported annually (1st group: 17.1/annum to 134/annum; 2nd group: 7.9/annum to 617/annum and 3rd group: 5.2/annum to 83.1/annum). The most common indication of the renal biopsy was a nephrotic syndrome.
The search comprised of articles from parts of Asia, Europe, and Africa. The highest prevalence among different types of glomerulopathy reported by all 35 studies was minimal change disease 16.5% (95% CI: 11.2-22.6; n=7657). Minimal change disease also reported in 2nd group by the study of Morocco was: 79.2% (68.5-87.6) on the other hand there were two studies from Nigeria which concluded minimal change disease the least common type found27,28. Minimal change disease was the highly prevalent type of pattern found in the pediatric group (3rd group) compared to group 1st and 2nd but no accountable difference was found across all the age groups (p=0.496).
The prevalence rate of focal segmental glomerulosclerosis in Africa was 15.9% (11.3-21.1). Among many regions, Focal segmental glomerulosclerosis (FSGS) was the most prevalent type of picture noted in West Africa: 19.1% (5.9-37.4) and it was the least common type in North Africa: 13.2% (9.5-17.5) but there was no significant inter regional difference noted (p=0.889). Other primary patterns of glomerulopathy were observed in 9.2% (95% CI: 6.2-12.7), among them mesangioproliferative glomerulonephritis 11.8% (95% CI: 9.2-14.6), Mesangiocapillary glomerulonephritis 6.6% (95% CI: 4.6-9.0) and membranous glomerulonephritis 2.8% (95% CI: 1.3-4.9). In the African continent, amyloidosis and lupus nephritis were two frequently reported diseases with a mean prevalence of 11.3% and 13.9% respectively.
In Asia, focal segmental glomerulosclerosis and minimal change disease were the commonest morphological variants. Minimal change disease was found in 35.48% of the studies (95% CI: 34.3-41.3). Focal segmental glomerulosclerosis was present among 37.58% of biopsies from Asia (95% CI: 37-38), membranous glomerulonephritis stood at 22.58% (95% CI: 4.1-0.9) and membranoproliferative glomerulonephritis was noted in 16.44% of the biopsies from the region (95% CI: 9.8-24.6). The most prevalent underlying condition in Europe was focal segmental glomerulosclerosis (28%), followed by minimal change disease (19.5%) and then membranous glomerulonephritis (18.5%), diabetic and hypertensive nephropathy, mirroring the high prevalence of diabetes and hypertension in the affluent parts of the world, were the predominant types of secondary renal diseases in Europe.
There is a scarcity of solid data regarding the epidemiology of glomerular diseases in many parts of the world in some measures due to the dearth of renal registries. Due to budget, insufficient skills to perform biopsies, tissue handling and adequate skills to interpret the morphology of renal tissue, especially in Africa and Asia. However, few institutions are efficient enough to perform renal biopsies regularly.
However, from what we gathered in this review, we have found that minimal change disease and focal segmental glomerulosclerosis are the frequently reported glomerular pattern in Asia,14 Europe and Africa. This may be because of nephrotic syndrome reported by the majority of the 29 studies as the only indication to perform a renal biopsy and as we noted in many studies these two patterns are the commonest cause of nephrotic syndrome29-32. We detected that there were very few studies33 which distributed the glomerulopathy in primary and secondary diseases. The most common secondary glomerulopathy found was lupus nephritis34-35.
Few researchers that have attempted to study secondary diseases, such as amyloidosis have shown that the deposition of amyloid is appeared in the mesangium as well as along the basement membrane of the glomerular capillary wall and this accumulation of amyloid damages the glomerular basement membrane. The presence of amyloid in terms of its quantity correlates with the clinical behavior of the disease as well. Among the histomorphological patterns, in lupus erythematosus, mesangial and membranous abnormalities can be found such as focal proliferative or diffuse proliferative mesangial or membranous glomerulonephritis, interstitial nephritis, glomerular sclerosis, vascular sclerosis and necrotizing renal vasculitis36.
Many different clinical characteristics and prognosis are associated with each of the morphological patterns. There is a possibility of occurrence of mesangial as well as focal proliferative lupus nephritis without the presence of any clinical feature and in general, they have a good prognosis. The progressive and irreversible renal functional abnormality and nephrotic syndrome are the manifestations of diffuse proliferative lupus nephritis. In one-sixth of the case, we observed milder forms could be transformed to diffuse proliferation. Although the nephrotic syndrome is the characteristic finding of membranous lupus nephritis, it may persistently present but the renal functional abnormality develops very slowly and its severity is least common37.
During diffuse proliferative lupus nephritis, necrotizing vasculitis occurs oftentimes and produces the picture of malignant hypertension which may ultimately lead to uremia. Interstitial nephritis occurs in the association of many glomerular patterns but most of the time it may occur as the predominant lesion both histologically and clinically as well. Glomerular sclerosis may occur with hypertension and vascular sclerosis majorly develops during lupus nephritis, and proceed towards severe forms even though the active entity has remitted38.
Hypertension also has its implications for kidney health and manifests important morphological signs as diseases progress. Microalbuminuria is a predictor of the clinical progression of the disease. Additionally, research also suggests that it is also a predictor of the clinical progression of diabetic nephropathy. The underlying histomorphological spectrum of both the disease is like that of the primary renal diseases39.
We recognized basic limitations in most of the studies including unavailability of immunofluorescence and electron microscopic technology and lack of uniformity in depicting morphological patterns. For example, we noted, many of the series published earlier in 2000 reported diffuse proliferative and focal proliferative glomerulonephritis even without further description of the picture. Although the performance of renal biopsy is mandatory for the appropriate diagnosis of glomerular diseases, we acknowledge, that this is not an impossible technique or method to establish and perform the renal biopsy in developing countries where the resources and competence are confined. Therefore, there is an urgent need to make pillars for the improvement in this area to perform, proceed and interpret the renal biopsy.
Table 1: Distribution of morphological patterns among different regions of the world.
| Research Study | Male | Female | Most Common PGN | Most Common SGN |
| Vuen et al. 2020 14 | 28.7% | 71.3% | MCD 38.9% | LN 87.2% |
| AlYousef et al. 2020 15 | 61.2% | 38.8% | IgA nephropathy 23.9% | LN 41.8% |
| Asif et al. 2017 33 | 53.3% | 646.6% | MN 71% | LN 60% |
| Ayach et al. 2011 34 | 61% | 39% | MCD 79.20% | Amyloidosis 2.6% |
| Nadium et al. 2013 36 | 54.9% | 45.1% | FSGS 29.6% | —– |
| Mohammad et al. 2012 | 76% | 24% | FSGS 22% | Amyloidosis 5% |
| Gunawardena et al. 2018 40 | 17.43 | 82.5% | FSGS 24.8% | 0% |
| Rathi et al. 2014 41 | 60.2% | 39.8% | FSGS 30.6% | LN 62.5% |
| PriyadarShini et al. 2019 42 | 62.97% | 37.03% | MCD 47.63% | LN (no % found) |
| MCD: Minimal change disease
FSGS: Focal segmental glomerulosclerosis LN: Lupus nephritis IgA: Immunoglobulin-A nephropathy |
||||
Glomerular diseases are an area of demanding expertise and the establishment of renal biopsy registries as these is found to be crudely portraying due to a lack of data on morphology. The step towards the betterment of this area may offer a chance to depict the prevalence and patterns of glomerulopathies and this may impacts positively on chronic kidney disease evaluation and treatment since most glomerular diseases are liable to treatment.
We are grateful to Miss Ambreen Wasim for her statistical guidance.
All the authors declare that there are no conflicting interests found in the preparation and publication of this research work.
AS wrote the manuscript, TM and FL revised and edited the manuscript.
- World Health Organization. World health statistics 2016: monitoring health for the SDGs sustainable development goals [Internet]. World Health Organization; 2016 [cited 2020 June 18]. Available from: https://apps.who.int/iris/handle/10665/206498
- Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, et al. Kidney-failure risk projection for the living kidney-donor candidate. New Eng J Med. 2016;374(5):411-421.
- Mubarak M, Kazi JI, Naqvi R, Ahmed E, Akhter F, Naqvi SA, et al. Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrol. 2011;16(1):87-92.
- Ali Jaffar Naqvi S. Nephrology services in Pakistan. Nephrol Dial Transplant. 2000;15(6):769-771.
- Ullah K, Butt G, Masroor I, Kanwal K, Kifayat F. Epidemiology of chronic kidney disease in a Pakistani population. Saudi J Kidney Dis Transpl. 2015;26(6): 1307-1310.
- Jha V. Current status of end-stage renal disease care in India and Pakistan. Kidney Int Suppl. 2013;3(2):157-160.
- Africa N, Coast12 I, Faso15 B. Regional disparities in etiology of end-stage renal disease in Africa. Saudi J Kidney Dis Transpl. 2013;24(3):594-595.
- Bethesda MD. United States Renal Data System: 2014 [internet]. Annual Data Report; 2014 [cited 2020 Nov 18]. Available from: https://adr.usrds.org/2020
- Cai G, Chen X. Etiology, comorbidity and factors associated with renal function decline in Chinese chronic kidney disease patients. J Am Soc Nephrol. 2011;22: A 183-A184.
- Ali ET, Abdelraheem MB, Mohamed RM, Hassan EG, Watson AR. Chronic renal failure in Sudanese children: aetiology and outcomes. Pediatr Nephrol. 2009;24(2):349-353.
- Sumaili EK, Krzesinski JM, Cohen EP, Nseka NM. Epidemiology of chronic kidney disease in the Democratic Republic of Congo: review of cross-sectional studies from Kinshasa, the capital. Nephrol Ther. 2010;6(4):232-239.
- Zhuo L, Wang H, Chen D, Lu H, Zou G, Li W. Alternative renal biopsies: past and present. Int Urol Nephrol. 2018;50(3):475-479.
- Menn-Josephy H, Lee CS, Nolin A, Christov M, Rybin DV, Weinberg JM, et al. Renal interstitial fibrosis: an imperfect predictor of kidney disease progression in some patient cohorts. Am J Nephrol. 2016;44(4):289-299.
- Vuen LA, Chee PH, Lojikip SL, Wei WK, Kheng G, Sia CF. Pattern of biopsy-proven renal disease in Sabah: A retrospective cross-sectional study over 3.5 years. Med J Malaysia. 2020;75(2):152-157.
- AlYousef A, AlSahow A, AlHelal B, Alqallaf A, Abdallah E, Abdellatif M, et al. Glomerulonephritis histopathological pattern change. BMC Nephrol. 2020;21:1-7.
- Ando D, Yasuda G. Circadian blood pressure rhythm is changed by improvement in hypoalbuminemia and massive proteinuria in patients with minimal change nephrotic syndrome. Cardiorenal Med. 2016;6(3):209-215.
- Zhu P, Zhou FD, Wang SX, Zhao MH, Wang HY. Increasing frequency of idiopathic membranous nephropathy in primary glomerular disease: A 10-year renal biopsy study from a single Chinese nephrology centre. Nephrol. 2015;20(8):560-566.
- Ratzel S, Cullinan SB. Nephrotic syndrome (NS), the association of gross proteinuria, hypoalbuminaemia, edema, and hyperlipidemia. Am J Hum Genet. 2015; 96(1):153-161.
- Chembo CL, Marshall MR, Williams LC, Walker RJ, Lynn KL, Irvine J, et al. Long-term outcomes for primary glomerulonephritis: New Zealand Glomerulonephritis Study. Nephrol. 2015; 20(12);899-907.
- Nylk J, Pullman JM, Campbell EC, Gunn-Moore FJ, Prystowsky MB, Dholakia K. Structured illumination microscopy as a diagnostic tool for nephrotic disease. In advanced biomedical and clinical diagnostic and surgical guidance systems XV. Int Soc Opt Photonics. 2017; 10054, p.1005417. Available from, https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10054/1005417/
- De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29(3):759-774.
- Krishnamoorthy S, Ramakrishnan SR, Vivek B, Suja L. Clinical, biochemical and histopathological profile of adult nephrotic syndrome patients in a tertiary care hospital. J Evol Med Dent Sci. 2015;4(71):12361-12376.
- Ikram M, Muhammad S, Ali A, Muhammad N, Bahadur K, Haq MI. Frequency of histopathological pattern of renal disease in a tertiary care hospital. J Postgrad Med Inst. 2017;31(2):131-134.
- Hommos MS, De Vriese AS, Alexander MP, Sethi S, Vaughan L, Zand L, et al. The incidence of primary vs secondary focal segmental glomerulosclerosis: A Clinicopathologic Study. Mayo Clin Proc. 2017;92(12):1772-1781).
- Wang H, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1084-1150.
- Joly D, Béroud C, Grünfeld JP. Rare inherited disorders with renal involvement—approach to the patient. Kidney Int. 2015;87(5):901-908.
- Fencl F, Vondrak K, Rosík T, Zieg J, Chadimová M, Háček J, et al. Recurrence of nephrotic proteinuria in children with focal segmental glomerulosclerosis: early treatment with plasmapheresis and immunoadsorption should be associated with better prognosis. Minerva Pediatr. 2016;68(5): 348-354.
- Cunningham A, Benediktsson H, Muruve DA, Hildebrand AM, Ravani P. Trends in biopsy-based diagnosis of kidney disease: a population study. Can J Kidney Health Dis. 2018;5:1-9.
- Wijkström J, González-Quiroz M, Hernandez M, Trujillo Z, Hultenby K, Ring A, et al. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis.2017;69(5):626-636.
- Hu W, Chen Y, Wang S, Chen H, Liu Z, Zeng C, et al. Clinical–morphological features and outcomes of lupus podocytopathy. Clin J Am Soc Nephrol. 2016;11(4):585-592
- Stanchev SS, Iliev AA, Malinova LG, Landzhov BV, Kotov GN, Hinova-Palova DV. Light microscopic study of renal morphological alterations in spontaneously hypertensive rats. J Biomed Clin Res. 2017;10(1):18-24.
- Lionaki S, Marinaki S, Panagiotellis K, Tsoumbou I, Liapis G, Vlahadami I, et al. Glomerular diseases associated with malignancies: histopathological pattern and association with circulating autoantibodies. Antib. 2020;9(2):18.
- Asif N, Ahsan MN, Khanzada SW. Spectrum of renal parenchymal diseases: An eleven year retrospective review of renal biopsy data from a tertiary care hospital in Pakistan. Ann King Edward Med Univ. 2017;23(1):13-17.
- Ayach G, El-Filali H, Saidi S, El-Gharib S, Kaobai G, Noaur H, et al. Histopathological study of pure primary nephrotic syndrome in adolescents and young Moroccan adults. Arab J Nephrol Transplant. 2011;4(3):137-140.
- Al-Sadoon EI, Rahim KA, Al-Anazi A, Faqeehi H, Al-Batati S. Spectrum of pediatricbiopsy-proven renal diseases: A single center experience. Saudi J Kidney Dis Transplant. 2020;31(1): 176-181.
- Nadium WK, Abdelwahab HH, Ibrahim MA, Shigidi MM. Histological pattern of primary glomerular diseases among adult Sudanese patients: A single center experience. Indian J Nephrol. 2013;23(3): 176-179.
- Kandil H, Sosnin D, Mahmoud A, Shalaby A, Soliman A, Elmaghraby A, et al. Hypertension and correlation to cerebrovascular change: A brief overview. Cardiovas Imaging Image Anal. 2018:345.
- Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, et al. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27(7):2135-2147.
- Mohammad N, Khan TM, Orakzai AN, Imran M. Histological pattern of glomerulopathies. Gomal J Med Sci. 2012;10(1):7-11.
- Gunawardena K, Wijewickrama E, Arambepola C, Lanerolle R. Descriptive analysis of glomerulonephritis by histological type and their progression among adults in a tertiary care center in Sri Lanka. Saudi J Kidney Dis Transplant. 2018;29(1): 136-139.
- Rathi M, Bhagat RL, Mukhopadhyay P, Kohli HS, Jha V, Gupta KL, et al. Changing histologic spectrum of adult nephrotic syndrome over five decades in north India: A single center experience. Indian J Nephrol. 2014;24(2): 86-91.
- PriyadarShini L, Pradhan SK. Pattern of renal histopathological findings in children: a single center study. J Clin Diagn Res. 2019;13(12)1-4.
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
