By Afsheen Khan1, Saif Ullah Shaikh2, Rabiya Ali3, Syeda Bushra Ahmed1
- Department of Anatomy, Shaheed Mohtarma Benazir Bhutto Medical College, Karachi, Pakistan.
- Department of Physiology, Bahria University Health Sciences, Karachi, Pakistan.
- Department of Physiology, Karachi Institute of Medical Sciences, CMH, Malir Cantt, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD12-2/005
How to cite: Khan A, Shaikh SU, Ali R, Ahmed SB. Age and Prostatic Volume: The Prognosis for Benign Prostatic Hyperplasia. Pak J Med Dent. 2022;12(2): 23-28. doi: 10.36283/PJMD12-2/005
Background: Old age is more susceptible to prostatic sicknesses. Benign prostatic hyperplasia (BPH) is related to repeated urinary tract infections, which influences personal satisfaction. The values of prostatic volume and obesity are viewed as significant factors for the advancement of prostate organ hypertrophy. The study aimed to investigate the effect of age on benign prostatic hyperplasia patients in a tertiary care hospital in Karachi.
Methods: A cross-sectional study was conducted in an emergency clinic of Dr. Ruth Pfau and Dow College of health sciences on 60 enrolled patients (50-80 years). All selected patients had BPH and were assessed by a Global prostate side effect score >7. Patients with BPH were divided into two groups obese and non-obese. Transrectal ultrasound was performed to analyze the length, width, diameter and volume of the prostate. The outcomes were then examined to observe the progressions in the morphological construction of the prostate and its relationship with the advancement of age.
Results: The mean prostate volume (PV) was higher in the obese group measuring 36.13±3.673ml while in the non-obese group, it was 31.21±6.771 ml, the difference was statistically significant (p=0.001). In the 60-70 years, age group, we obtained the highest count of obese participants with PV ≥ 30ml. In 71-80 years, age groups, again the maximum number of participants had ≥ 30ml PV and were obese.
Conclusion: In the study, patients’ obesity had a significant effect on the prostate volume (p<0.001). Thus, benign prostatic hypertrophy was seen in the higher age group (60-70 years).
Keywords: Old Age, BPH, Prostatic Volume, Satisfaction.
Advanced age is more prone to prostatic diseases. Benign Prostatic Hypertrophy (BPH) is one of the major health concerns for aging males. It is associated with lower urinary tract symptoms, which affects the quality of life. Obesity and androgenic hormones (estrogen) are regarded as important risk factors for the development of prostate gland hypertrophy. BPH is a progressive disease and age is the biggest risk factor for its advancement. Studies have shown that the Prostate Volume of BPH patients differs not only with age but is affected by some variables of obesity too. BPH has been linked to metabolic disorders such as obesity1. Raza et al. reported that Asian men’s PV is lower than that of white men in their study, which showed racial differences in PV2. According to the literature, PV varies across different racial communities3.
Current research has shown that Prostate Volume (PV) has significant clinical effects related, to the pathologies of the prostate. The changes in prostatic volume are common throughout the man’s life2. Like some other longitudinal studies, total prostate volume is observed to be enlarged and 3.5% per year is analyzed in the transition zone. Prostate volume measurement has much importance for future enlargement in many clinical settings2. New data has observed that men who had less than 30ml of prostatic volume showed 1.7% growth in median prostate per year; nevertheless, those have more than 30ml of PV presented median prostate growth of 2.2% per year 1. In young males, the peripheral zone (PZ) central zone (CZ) and transitional zone (TZ) constitute 75, 20 and 5% of the gland volume but these ratios change with age and the onset of benign prostatic hypertrophy (BPH). BPH starts in the TZ and can ultimately occupy most of the gland3. Most voiding problems in elderly men are due to BPH 4. It is a non-malignant growth of the prostate. It is considered the fourth most prevalent disease in men aged more than 50 years5. Its frequency is about 40% in men aged 50- years or above and about 90% in men aged 80 years or above6. The study aimed to relate the prostatic volume and obesity with age advancement in known adults with BPH.
This cross-sectional study was carried out on sixty (60) diagnosed cases of BPH from the emergency clinic of Dr. Ruth Pfau and Dow University of health sciences. The age of the study population ranged from 50-80 years. Individuals with an international prostatic symptom score (IPSS) less than 7 were considered as healthy. Patients who scored more than 7 were included in the study whereas those with a known case of prostate carcinoma, renal and bladder stones, or any type of pelvic and prostatic surgery were excluded from the study.
The sample size estimation was done through the open Epi software, calculated at 95% confidence interval and 80% power of the test, all patients were categorized into 2 groups obese and non-obese based on body mass index and waist circumference to make a total of 60. Transrectal ultrasound (TRUS) was performed on all patients using an Ultrasound machine named (Type Doppler machine and Toshiba company model Nemio XG) for measuring the size and volume of the prostate. The width was measured between the inner part of the capsule, the height from the bladder neck to the clear inferior limit and the length from the inner of the capsule to the clear limit of the transition zone at the verumontanum7. The transitional zone was scanned in transverse and sagittal planes with the subject in the left lateral decubitus position by ellipsoid formula (height × length × width π/6) to measure the prostate gland dimensions (length, width, and depth) and to derive the volume of the prostate gland8. TRUS has a vital role in urology because of its diagnostic and therapeutic potential and was first developed in the 1970s 9. It is a gold standard, reliable and safe clinical tool for the assessment of prostatic size3. Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 21.0. Descriptive statistics including mean, standard deviations, frequency and proportions were calculated for baseline characteristics of obese and non-obese participants. Kruskal Wallis and Mann-Whitney tests were used to associate the mean differences between age groups. p-values < 0.05 were considered significant.
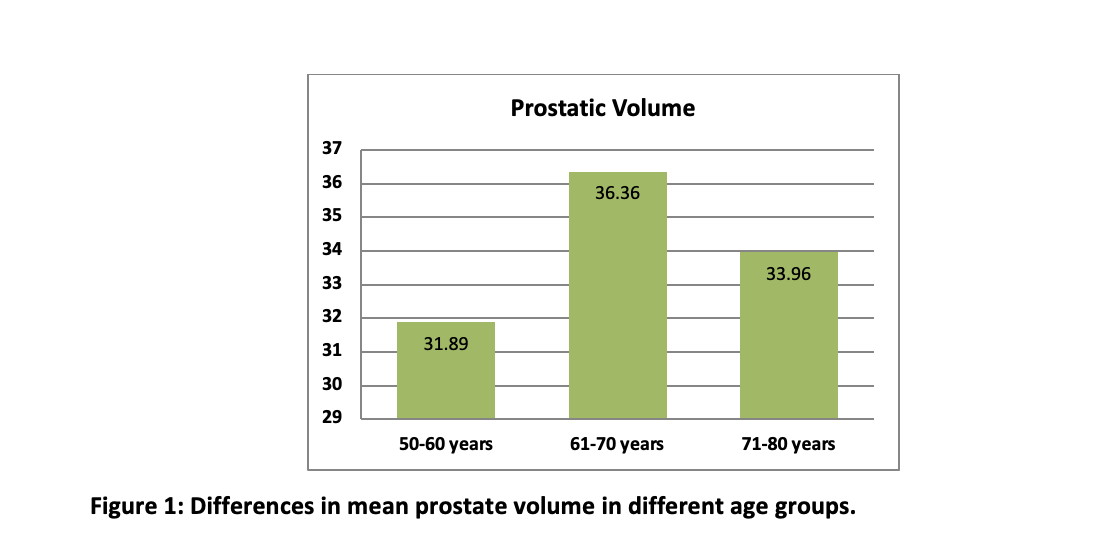
Among n=60 patients 65 ± 9.98 years was the mean age (range 5-80 years). The study population was distributed into two groups, obese and non-obese, each comprising 30 individuals Age was classified 1, 2, 3 i.e. (50-60), (61-70) and (71-80). The majority of 23 (38.3%) ranged between 71-80 years of age, while 18 (30%) participants were between 61-70 years of age and 19 (31.7%) participants were aged between 50-60 years. We obtained prostate volume (mean ± SD) of 31.89±5.606 in the age group 50-60 years, 36.36±4.11 in the age group 61-70 years and 33.96±7.119 in the age group 71-80years. When the prostate volume of different age groups was analyzed, a statistically significant difference was obtained in PV according to their ages (p=0.05) (Figure 1). The highest mean PV was found in 60-70 years i.e., middle age. The demographic characteristics highlighted mostly participants non-educated (91.7%), Urdu (37%) and Punjabi (15%) ethnicity and the low class 68% (Table 1).
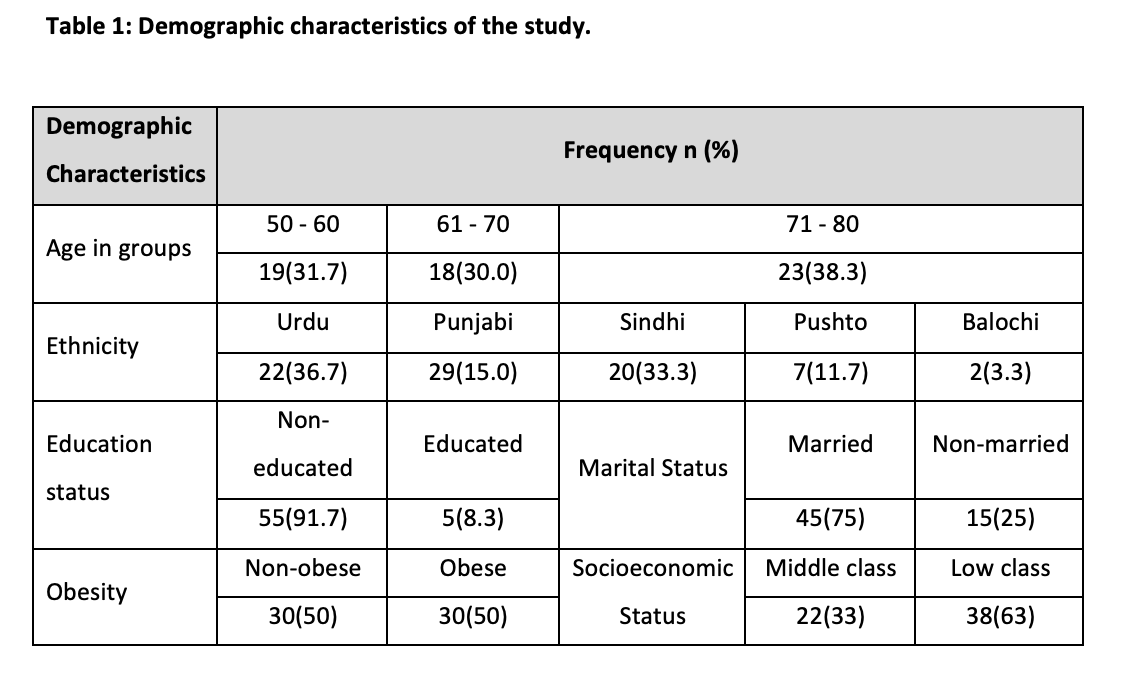
Out of 60, 47 participants were classified as having prostate volume (PV) ≥ 30 ml with a high percentage of 78.3%, while 13 men were classified as having PV < 30 ml with a percentage of 21.7. Serum estrogen levels were found to be ≥ 192 in 52 participants (86.7%) while 8 participants (13.3%) had < 192. The characteristics of study participants stratified according to Obesity are shown in Table 2. Distribution of participants, according to the frequency of prostate volume below and above the cutoff values i.e. (<30ml and ≤ 30ml) in obese and non-obese participants of different age groups (Table 2). Insignificant results were found in all groups. In the age group, 50-60 maximum numbers of participants were non-obese with PV ≥ 30ml. In the 60-70 years, age group, we obtained the highest count of obese participants with PV ≥ 30ml. In 71-80 years, age groups, again the maximum number of participants had ≥ 30ml PV and were obese. The relationship between age, obesity and PV was not significant (Table 2).
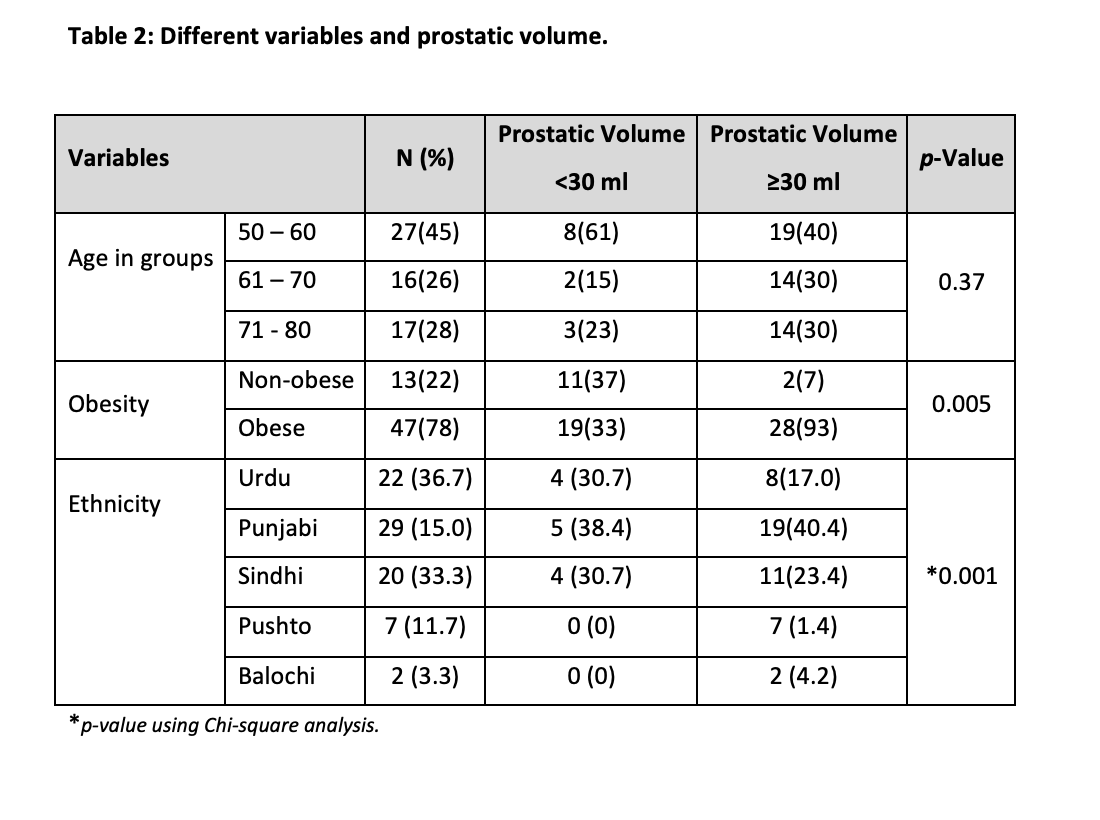
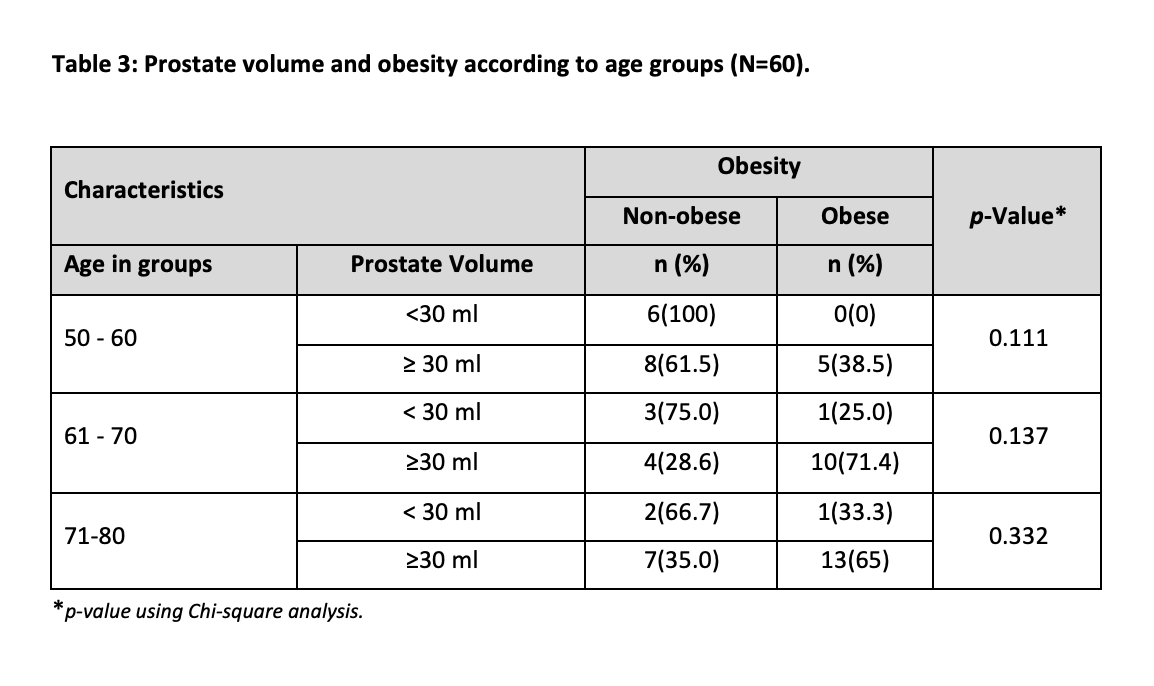
Out of 60, 47 participants were classified as having prostate volume (PV) ≥ 30 ml with a high percentage of 78.3%, while 13 men were classified as having PV < 30 ml with a percentage of 21.7. Serum estrogen levels were found to be ≥ 192 in 52 participants (86.7%) while 8 participants (13.3%) had < 192. The characteristics of study participants stratified according to Obesity are shown in Table 2. Distribution of participants, according to the frequency of prostate volume below and above the cutoff values i.e. (<30ml and ≤ 30ml) in obese and non-obese participants of different age groups (Table 2). Insignificant results were found in all groups. In the age group, 50-60 maximum numbers of participants were non-obese with PV ≥ 30ml. In the 60-70 years, age group, we obtained the highest count of obese participants with PV ≥ 30ml. In 71-80 years, age groups, again the maximum number of participants had ≥ 30ml PV and were obese. The relationship between age, obesity and PV was not significant (Table 2).
Age, ethnicity, obesity, genetics, sex steroid hormones, modifiable lifestyle variables, and inflammation are known stimulators of morphological volumetric changes in the prostate gland. Age is one factor that might cause changes in the prostate gland10. In this study, we found significant results in the age range of 61-70 years and prostate volume of 30ml when comparing obese and non-obese patients. Therefore, lack of exercise, sedentary lifestyles, poor food, and smoking all contribute to prostate enlargement, which is most likely to be the source of high values in obese participants in the age range of 51 to 60 years.
It is a very significant issue, for every year, there is a 4% increase in prostate size. The prostate gland grows at different rates in different age stages. The structural changes in the prostate gland due to increased age and the growth rate was calculated to be 0.81 % per year corresponding to 0.2 ml per year. The age distribution of study participants in our study is from 50 to 80 years. The maximum number of study participants being observed in their seventh decade (38.3%). It is well-established that there is a specific link between prostatic growth with advanced age11. Advanced ages cause urological problems, which can affect the quality of life significantly. Throughout a man’s lifetime, his prostate glands grow it is well known that prostatic volume can indicate future growth and development12,13.
Study participants were recruited because of IPSS scores greater than 7, so a volume greater than 30 is susceptible according to their enlargement. The highest prostate volume observed in our study is 42ml. In contrast, research conducted in Pakistan by Raza et al. observed 103 benign prostatic hypertrophic (BPH) participants, out of which 80 participants had PV lower than 50ml while 23 participants had greater than 50ml. 90ml was the maximum prostate volume in their study2,14. In addition to this, another study observed 65.6% of the patients had a prostate volume between 25 to 50ml and 35% of the participants had a prostate volume of more than 50ml and Kim et al15. Alongside, another study reported the highest number of patients had prostate volume ranging from 20 to 50ml 16. Similarly, 79% of patients in the Indian study reported between 25 to 50ml prostate volume17. The evaluation by volume analysis in cases of prostate enlargement is significant in several cases. Both the disease’s progression and its complications can be seen. Several investigations have revealed that the prostatic volume varies in obese healthy individuals18.
In research done on a similar population to our study, 103 participants found a relation between prostate volume (PV) with obesity and reported that mean prostate volume was 36ml in non-obese and 54ml in obese1. The positive association between obesity and prostate volume (PV) was also observed by other researchers showing19,20. The linear relationship between the two variables. Our results are consistent with the study mentioned. It could be due to the fat deposition which causes the adipose tissue to accelerate the aromatization of circulating testosterone into estrogens which influences the prostate volume21.
Urological issues brought on by old age can significantly lower the quality of life. Canning et al.22 found that 30% of the population showed moderate to severe symptoms of the lower urinary tract in people older than 50. The most prevalent symptoms in males older than 70 years were nocturia and frequency of urination in men younger than 70 years23. It is well known that the prostatic volume can predict later development and growth24. When comparing obese and non-obesity patients in our study based on age and prostate volume, we found significant outcomes in the age group of 61-70 years and prostate volume of 30ml.
In contrast to our findings, earlier research has shown that prostatic enlargement occurs in 44.0% of participants who are 80 years or older, with a significant difference between obese and non-obese persons 21. Like this, those who were obese and in older age groups had higher prostatic volumes23. Several investigations discovered that the prostate gland grew larger with age and that there was a strong association between the size of the prostate gland and age 25. Most studies found that men over 70 had the largest mean prostate volumes2. Our results show that the mean PV increases between the ages of 61 and 70, suggesting that our population has been subjected to risk factors (obesity, high androgenic hormones, and dietary variables) that have resulted in higher PV (36.36+4.11) at a very young age. Despite any obvious differences, the age group 50–60 years had the largest number of non–obese participants with PV 30ml. But most individuals in the age ranges of 61 to 70 and 71 to 80 were obese with PV 30ml. This evidence supports many other studies’ conclusions that different age groups with high PV and obesity differ significantly from one another22.
In comparison to the non-obese group within the same age range, a significant positive correlation between prostate volume and obesity was found. Obesity significantly affected the prostate volume in the study patients and consequently benign prostatic hypertrophy. The associations between prostate hyperplasia and aged men need additional investigation in longitudinal research.
The authors appreciate the cooperation of their professors and senior colleagues for their guidance.
There was no conflict of interest among the authors.
The study was approved by the ethics review committee of the organization.
Written consent was taken from the parents of all participants. Anybody who wished to drop in the middle of the study was allowed to do so.
AK and SUS were involved in conceiving the idea, benchwork and writing the manuscript. RA did the statistical analysis. SBA and SUS worked on manuscript writing and proofreading.
- Williams N, O’Connell PR, editors. Bailey & Love’s short practice of surgery. CRC press; 2008, p. 1343.
- Raza I, Hassan N, Gul P, Jafri A, Zehra N, Younas N. Determination of prostate gland volume by ultrasonography and its correlation with anthropometric measurements in a subset of Karachi population. Br J Med Med Res. 2016:1-12. doi: 10.9734/BJMMR/2016/20530
- Harvey C, Pilcher J, Richenberg J, Patel U, Frauscher F. Applications of transrectal ultrasound in prostate cancer. Br J Radiol. 2012 Nov; 85(Spec Iss 1): S3-S17. doi: 10.1259/bjr/56357549
- Chute C, Panser L, Girman C, Oesterling J, Guess H, Jacobsen S, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urology. 1993;150(1):85-89. doi: 10.1016/S0022-5347(17)35405-8
- Issa MM, Regan TS. Medical therapy for benign prostatic hyperplasia–present and future impact. Am J Manag Care. 2007;13:S4-S9.
- Lee G, Shin J, Choi H, Jo A, Pan S, Bae D, et al. Cynanchum wilfordii ameliorates testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase and androgen receptor activities in a rat model. Nutrients. 2017;9(10):1-15. doi: 10.3390/nu9101070
- Xie LP, Bai Y, Zhang XZ, Zheng XY, Yao KS, Xu L, et al. Obesity and benign prostatic enlargement: a large observational study in China. Urology. 2007;69(4):680-684. doi: 10.1016/j.urology.2006.12.030
- Fujita K, Hayashi T, Matsushita M, Uemura M, Nonomura N. Obesity, inflammation, and prostate cancer. J Clin Med. 2019;8(2):1-15. doi: 10.3390/jcm8020201
- Mitterberger M, Horninger W, Aigner F, Pinggera GM, Steppan I, Rehder P, Frauscher F. Ultrasound of the prostate. Cancer Imaging. 2010; 10(1): 40-48. doi: 10.1102/1470-7330.2010.0004
- Afroz T, Sultana N, Rahman M, Begum A, Muna F, Rahman M. Association between metabolic syndrome and benign prostate hyperplasia. Bangladesh J Med Biochem. 2015;8(2):42-48. doi: 10.3329/bjmb.v8i2.33278
- Jung JH, Ahn SV, Song JM, Chang SJ, Kim KJ, Kwon SW, et al. Obesity as a risk factor for prostatic enlargement: a retrospective cohort study in Korea. Int Neurourol J. 2016;20(4):321-328. doi: 10.5213/inj.1632584.292
- Gómez-Ambrosi J, Silva C, Galofré J, Escalada J, Santos S, Millán D, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. 2012;36(2):286-294. doi: 10.1038/ijo.2011.100
- Ho EL, Tong SF, Tan HM. Prostate size: Is size all that matters?(When does size matter?). J Mens Health. 2011;8:S22-S24. doi: 10.1016/S1875-6867(11)60014-6
- Naz Z, Anjum S. Effect of anthropometric measurements and personal data parameters on benign prostatic hyperplasia and carcinoma prostate. J Ayub Med Coll Abbottabad. 2010;22(3):54-57.
- Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity. 2007;15(1):216-216. doi: 10.1038/oby.2007.505
- Mondal N, Timungpi R, Kathar M, Hanse S, Teronpi S, Timung A, et al. Cut-off point estimation of neck circumference to determine overweight and obesity among Asian Indian adults. Epidemiol Biostat Public Health. 2017;14(2):1-9. doi: 10.2427/12310
- Deurenberg P, Deurenberg‐Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141-146. doi: 10.1046/j.1467-789X.2002.00065.x
- Kim GW, Doo SW, Yang WJ, Song YS. Effects of obesity on prostate volume and lower urinary tract symptoms in Korean men. Korean J Urol. 2010;51(5):344-347. doi: 10.4111/kju.2010.51.5.344
- La Vignera S, Condorelli R, Russo G, Morgia G, Calogero A. Endocrine control of benign prostatic hyperplasia. Andrology. 2016;4(3):404-411. doi: 10.1111/andr.12186
- Lee S, Min HG, Choi SH, Kim YJ, Oh SW, Kim YJ, et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity. 2006;14(1):172-179. doi: 10.1038/oby.2006.21
- Lee RK, Chung D, Chughtai B, Te AE, Kaplan SA. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 2012;110(4):540-545. doi: 10.1111/j.1464-410X.2011.10819.x
- Jakobsen H, Torp-Pedersen S, Juul N. Ultrasonic evaluation of age-related human prostatic growth and development of benign prostatic hyperplasia. Scand J Urol Nephrol Suppl. 1988;107:26-31.
- Alanazi AB, Alshalan AM, Alanazi OA, Alanazi MS, Alanazi AI, Alanazi AH, et al. Epidemiology of senile prostatic enlargement among elderly men in Arar, Kingdom of Saudi Arabia. Electron Physician. 2017; 9(9): 5349-5353. doi: 10.19082/5349
- Ng TP, Jin A, Chow KY, Feng L, Nyunt MSZ, Yap KB. Age-dependent relationships between body mass index and mortality: Singapore longitudinal ageing study. PLoS One. 2017;12(7):1-11. doi: 10.1371/journal.pone.0180818
- Canning KL, Brown RE, Jamnik VK, Kuk JL. Relationship between obesity and obesity-related morbidities weakens with aging. J Gerontol A Biol Sci Med Sci. 2014;69(1):87-92. doi: 10.1093/gerona/glt026
- Basawaraj N, Arul D, Ashok K, Srinath M. Can sonographic prostate volume predict prostate specific antigen (PSA) levels in blood among non prostatic carcinoma patients. Int J Biol Med Res. 2012;3(3):1895-1898.
- Yim SJ, Cho YS, Joo KJ. Relationship between metabolic syndrome and prostate volume in Korean men under 50 years of age. Korean J Urol. 2011;52(6):390-395. doi: 10.4111/kju.2011.52.6.390
- Timms BG, Hofkamp LE. Prostate development and growth in benign prostatic hyperplasia. Differentiation. 2011;82(4-5):173-183. doi: 10.1016/j.diff.2011.08.002
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
