By Sidrah Mahmood Mughal1, Mervyn Hosein2, Sana Mirza3, Moazzam Ali Shahid4, Aiza Suhail Khan5
- Department of Oral Pathology, Ziauddin College of Dentistry, Karachi, Pakistan.
- Ziauddin College of Dentistry, Karachi, Pakistan.
- Department of Oral Pathology, Ziauddin College of Dentistry, Karachi, Pakistan.
- Department of Research, Ziauddin College of Dentistry, Karachi, Pakistan.
- Department of Oral and Maxillofacial Surgery, Liaquat National Hospital, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD12-1/008
How to cite: Mughal SM, Hosein M, Mirza S, Shahid MA, Khan AS. Role of Salivary Chemerin in Oral squamous cell carcinoma (OSCC) Patients with and without Chronic Periodontitis. Pak J Med Dent. 2023;12(1): 38-45. doi: 10.36283/PJMD12-1/008
Background: Chemerin is a multifunctional protein that plays a role in immunological function, chemotaxis, energy metabolism, and basic cell activities. Chemerin dysregulation contributes significantly to tumor angiogenesis and disease progression. The study aimed to assess salivary chemerin levels in Oral squamous cell carcinoma (OSCC) patients with/without chronic periodontitis, as well as, investigate the relationship between chemerin levels and OSCC stages.
Methods: This case-control study was conducted at the Dental OPD of Ziauddin University Hospital and Liaquat National Hospital Karachi Pakistan from January 2020-2021. A total of n=60 participants, 15 each of OSCC with and without periodontitis and 15 controls of periodontitis alone and 15 healthy controls were included in the study. The salivary levels of chemerin were measured by ELISA. The mean salivary chemerin level was compared using Kruskal–Wallis’s test among the stages, sites and grades of OSCC. A p-value <0.05 was considered statistically significant.
Results: The mean age of study participants was estimated as 43.42±13.19 years (range: 20-60 years). The mean salivary chemerin levels in OSCC were highest in the late stages; 18.88±8.8 ng/ml in stage III and 17.63±5.09 ng/ml in stage IV. A statistically significant difference was observed in mean salivary chemerin levels concerning the stage of OCSS (p=0.025). Furthermore, periodontal status was statistically associated with the site of the tumor (p<0.05).
Conclusion: In OSCC and periodontitis, salivary chemerin levels were shown to be higher compared to healthy controls (p=0.025). Therefore, it can be utilized as a new therapeutic target for detecting tumorigenesis.
Keywords: Chemerin; Oral Squamous Cell Carcinoma; Periodontitis; Periodontal Disease; Saliva; Salivary Chemerin Level.
Oral Squamous Cell Carcinoma (OSCC) is the most frequent cancer in the world, with more than 0.5 million new cases identified each year with the worst prognosis1. Despite recent advancements in cancer prevention and treatment measures, OSCC has a five-year survival rate of 50-62% 2,3. Tobacco (chewable and smoking), alcohol, and poor oral hygiene, which leads to periodontitis, which promotes oncogenesis and the formation of oral cancer, are the most prevalent risk factors for OSCC1,4,5.
Emerging data and literature also suggest a very strong association between oral cancer and periodontitis, with chronic inflammation being a key contributory component in both diseases6. Chronic periodontitis is characterized by chronic inflammation in which microorganisms activate a variety of host cells, including neutrophils, monocytes, macrophages, lymphocytes, epithelial cells, and fibroblasts, to produce reactive nitrogen and oxygen species, metabolites, and matrix metalloproteinase capable of inducing DNA damage in epithelial cells; and secondly, to produce cytokines, chemokines, and growth factors that can provide an atmosphere conducive to survival, proliferation and migration of cells, angiogenesis, and inhibition of apoptosis leading towards carcinogenesis7.
Recently, it has been reported that a few adipokines including chemerin, released from adipose tissue and the defense cells in the tissue are involved in the inflammatory regulation and response8. Moreover, increased salivary chemerin levels of patients suffering from periodontitis were reported, which could be linked to the destruction of periodontal tissues9. Chemerin, a multifaceted protein, has roles in immune function, chemotaxis, energy metabolism along with fundamental cell processes10. It is involved in the regulation of tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) 8. Interestingly, many kinds of cancer have unregulated chemerin expression, according to the literature. Chemerin levels were shown to be higher in individuals with colon cancer11, stomach cancer12, and grade III/IV gliomas13. Further, in the study by Wang et al., the protein and chemerin mRNA overexpression was correlated with tumorigenesis and poor progression in tongue squamous cell carcinoma14. Hence, this evidence showed that the deregulation of chemerin levels plays a significant part in tumor angiogenesis and disease progression10.
Unfortunately, there is a huge gap in the current Pakistani literature regarding the predictive role of salivary chemerin in OSSC and periodontitis. Salivary chemerin is a reliable and cost-effective biomarker for the diagnosis and screening of high-risk individuals. Moreover, since tobacco and alcohol are commonly attributed to causing OSCC, there is a worrisome trend of incidence of oral cancer in patients who have never used these etiological factors (tobacco and alcohol), suggesting the presence of other risk factors causing tumor formation. Epidemiologic evidence supports the underlying role of periodontal disease causing the risk of OSCC via the immune inflammatory pathway. Hence, by investigating the levels of an inflammatory biomarker in OSCC patients and healthy controls, we will be able to define cut-off values for high-risk people. Therefore, this research aimed to study the expression of salivary chemerin in OSCC patients with and without chronic periodontitis and to find the association between chemerin levels and various stages of OSCC.
It was a case-control study conducted at the Dental OPD of Ziauddin University Hospital and the Dental OPD of Liaquat National Hospital Karachi Pakistan for one year. The sample size was calculated on OpenEpi (software) at 95% confidence level and 80% power of the test, by taking statistics of mean salivary chemerin level for the OSCC group as 13.19 ± 3.75 ng/ml and Control group as 3.06 ± 0.69 ng/ml were taken15. The total sample size calculated was 40, which was increased to 60 for more statistically relevant results i.e., thirty in cases and thirty in controls (15 cases of OSCC with periodontitis, 15 cases of OSCC without periodontitis, 15 controls of periodontitis alone along with15 healthy controls were included in the study). Inclusion criteria for cases involved male and female patients between 20 to 60 years of age. Untreated OSCC, all stages, based on clinical examination and confirmed by histopathologist and OSCC patients with and without periodontitis were included, whereas male and female patients in the 20 to 60 years of age group and individuals with and without periodontitis were recruited to meet the inclusion criteria for disease-free controls. Exclusion criteria for cases and disease-free controls involved patients having chronic inflammatory systemic diseases or with a history of (prior) malignancy, autoimmune diseases, immunodeficiency-associated disorders and hepatitis. Patients who had prior treatment in the form of chemo- and radiotherapy, alternative medicine, or surgery for a prior malignancy, obese patients and present or former user of any kind of tobacco products.
Ethical approval was obtained from the Ethics Review Committee of Ziauddin University. Informed consent was obtained from each participant of the study. Proforma and questionnaire having information on demographic profile and variables like age, gender and characteristics of OSCC determined by the biopsy report were filled by the principal investigator. An assessment of periodontitis was performed. Whole un-stimulated saliva (WUS) was collected using standard techniques16. Samples were centrifuged for thirty minutes at 1,000×g. The supernatant was collected and assayed immediately, or stored in an aliquot at -80 °C for use later. The salivary levels of chemerin were measured for both groups, using the (SEA945Hu) Human Chemerin ELISA, Cloud Clone Corp. USA. The kit is a sandwich enzyme immunoassay for quantitative measurement (in vitro) of chemerin in human plasma, serum, cell lysates, tissue homogenates, cell culture supernatant as well as other, biological fluids. During the reagent preparation samples were kept at room temperature 18-25 ℃ and diluted standards were prepared at different concentrations such as 10ng/mL, 5ng/mL, 2.5ng/mL, 1.25ng/mL, 0.625ng/mL, 0.312ng/mL and 0.156ng/mL. The detection range was 0.156-10ng/mL.
SPSS software version 23 was used for the analysis of data. The normality of the numeric data was assessed using the Shapiro-Wilk test. The p-value of the Shapiro-Wilk Test greater than 0.05 showed that the data was normally distributed. The numeric variables i.e., age and salivary chemerin levels were presented as mean and SD or median and interquartile range. The categorical variables i.e., gender, site, stage and grade of tumor were presented as frequency and percentage. The distribution of salivary chemerin level was non-parametric among the patients with OSCC therefore, the mean of salivary chemerin level was compared using Kruskal–Wallis’s test among the stages, sites and grades of OSCC. Fisher’s exact test was applied for comparison of the site of the tumor with stage, grade of tumor and periodontal status. A p-value < 0.05 was considered statistically significant.
A total of 60 patients were included in the study. The overall mean age of study participants was estimated as 43.42±13.19 years (range: 20-60 years). The mean age of patients with OSCC was reported as 50.67±8.57 years (range: 27-70 years) and without OSCC was reported as 36.17±13.11 years (range: 20-64 years). Further, the mean age of OSCC patients with periodontitis was 52.53±9.68 years (Figure 1a-d). Gender-wise distribution of OSCC patients, OSCC + periodontitis patients, without OSCC + periodontitis and healthy controls is displayed in Figure 2 and 3.
Figure 1(a-d): The overall mean age of studied participants included the Oral squamous cell carcinoma (OSCC) group and healthy controls.
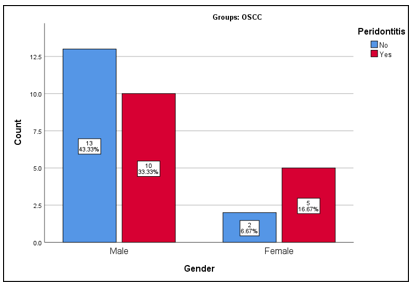
Figure 2: Frequency distribution of gender of patients with and without periodontitis in the Oral squamous cell carcinoma (OSCC) group.
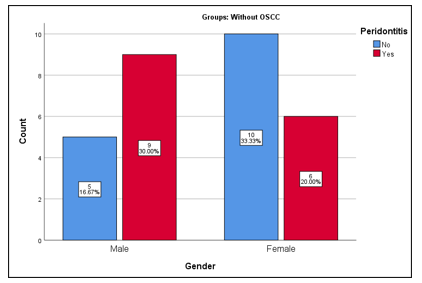
Figure 3: Frequency distribution of gender of patients with and without periodontitis in without Oral squamous cell carcinoma (OSCC) group.
Among patients with OSCC (n=30), most of the patients had a lesion on the buccal mucosa, so this was the common site of cancer among our study group 43%, 33% of the patients presented with Stage I of cancer and 50% had well-differentiated tumors. The mean salivary chemerin level was highest in the late stages of OSCC i.e., in stage III (18.88±8.8 ng/ml), followed by stage IV (17.63±5.09 ng/ml). A statistically significant difference was observed in mean salivary chemerin levels concerning the stage of OCSS (p=0.025). Whereas, no association was observed between the site and grade of the tumor with salivary chemerin level (Table 1).
Table 1: Comparison of salivary chemerin levels with the site, stage and grade of tumor in patients with Oral squamous cell carcinoma (OSCC).
| Characteristics of Oral Squamous Cell Carcinoma (OSCC) | Salivary Chemerin Levels | p-Value | |
| Median (IQR) | |||
| Site | Alveolar Ridge (n=5, 16%) | 18.34 (12.45-18.45) | 0.06 |
| Buccal Mucosa (n=13, 43%) | 14.49 (9.97-26.48) | ||
| Maxilla (n=2, 6.6%) | 12.05 (11.05-13.05) | ||
| Soft Palate (n=1, 3.3%) | 17.24 (17.08-17.40) | ||
| Tongue (n=9, 30%) | 8.66 (7.68-9.89) | ||
| Stage | I (n=10, 33%) | 8.76 (7.05-10.35) | 0.025* |
| II (n=8, 26%) | 12.39 (11.05-13.29) | ||
| III (n=6, 20%) | 18.14 (10.45-28.39) | ||
| IV (n=6, 20%) | 18.34 (17.40-18.45) | ||
| Grade | Moderately differentiated (n=13, 43%) | 12.34 (9.49-18.45) | 0.958 |
| Well differentiated (n=15, 50%) | 12.45 (9.57-16.89) | ||
| Well to moderately differentiated (n=2, 6.7%) | 13.44 (7.68-19.20) | ||
Table 2 displays the site versus stage, site versus grade and site versus the periodontal status of OSCC patients. There was no significant relationship observed between the stage and site of the tumor (p>0.05) and grade and site of the tumor (p>0.05), whereas, periodontal status was statistically associated with a site of the tumor (p<0.05).
Table 2: Comparison of the site of Oral squamous cell carcinoma (OSCC) with stage, grade of tumor and periodontal status.
| Variables | Alveolar Ridge | Buccal Mucosa | Maxilla | Soft Palate | Tongue | p-Value |
| Stage | ||||||
| I | 2 | 3 | 0 | 0 | 5 | 0.343 |
| II | 1 | 3 | 2 | 0 | 2 | |
| III | 0 | 3 | 0 | 1 | 2 | |
| IV | 2 | 3 | 0 | 1 | 0 | |
| Grade | ||||||
| Moderately Differentiated | 3 | 4 | 0 | 2 | 4 | 0.245 |
| Well Differentiated | 2 | 8 | 2 | 0 | 3 | |
| Well to moderately Differentiated | 0 | 0 | 0 | 0 | 2 | |
| Periodontitis | ||||||
| Negative | 1 | 6 | 0 | 0 | 8 | 0.013* |
| Positive | 4 | 6 | 2 | 2 | 1 | |
Oral squamous cell carcinoma (OSCC) patients mostly remain undiagnosed until the late stage, which is one of the prime causes of poor survival rate17,18. Therefore, the early detection of OSCC is important as the provision of therapy at an early stage of the tumor offers an improved prognosis and a higher probability of cure17,18. Standard methods for detecting oral malignancies include costly biochemical tests, clinical evaluation, and invasive biopsy19. In recent years, the use of salivary biomarkers for the early diagnosis of oral cancer, in the search for novel clinical indicators, has been a promising strategy due to the ease of collection and non-invasive sampling procedures19. Evidence has shown that many of the salivary biomarkers show significant levels in OSCC patients as compared to healthy controls2,19-22. To the best of our knowledge, this is the first study conducted among Pakistani individuals in which salivary levels of chemerin are examined in patients with OSCC, periodontitis, OSCC + periodontitis and healthy controls and also the evaluation of the association between chemerin levels and clinicopathological parameters of OSCC with clinical parameters of chronic periodontitis have been made.
Since chemerin is an inflammatory biomarker, the levels in saliva are raised in periodontitis8, 23. In a comparative study, the levels of three adipokines in healthy, gingivitis and periodontitis groups were assessed. The salivary progranulin levels were similar in all groups; salivary levels of visfatin were the same in the gingivitis and periodontitis groups; whereas the chemerin levels were higher in the periodontitis group with a value of 0.084 (0.063–0.105 median), as compared to the other two groups gingivitis (0.042) and the healthy group (0.042), and this was correlated with the degree of tissue destruction8. In addition, serum chemerin levels are also found to be useful in determining periodontal health24. In this study as the number of periodontally involved patients of OSCC and without OSCC is equal, there is no such association found between the risk of OSCC and periodontitis (OR=1.0, 95% CI: 0.36 to 2.75). However, literature shows that patients with periodontitis are at greater risk of OSCC as compared to healthy controls (Adjusted OR=3.7, 95% CI: 1.46 to 9.23) 25. Hence, the odds of OSCC could be modulated by decreasing periodontitis.
Many researchers have highlighted that chemerin plays a vital role in the development and progression of cancer26-29. In the present study, we observed that the salivary chemerin level was higher in the advanced stages of OSCC (Stage III-IV) than in the early stages (Stage I-II). A few researchers have also suggested that greater chemerin concentrations are extremely effective in detecting active carcinogenesis in the oral cavity and other areas of the body30. Similarly, in the current study, we found the level of salivary chemerin was greater in patients with cancer of the oral cavity than in patients without oral cancer. Comparable research by Ghallab et al. and Lu et al. also showed that the salivary chemerin level was substantially higher in OSCC patients as compared to healthy controls. They also found that salivary chemerin is 100% sensitive and specific in detecting OSCC at an early stage22,29. Hence, these findings indicate that chemerin could serve as a novel biomarker for OSCC patients since early diagnosis and initiation of treatment can be done before the disease progresses further.
Chemerin levels in saliva are higher in patients with periodontal disease, and since periodontal disease is linked to an imbalance of the oral microbiota, chemerin could influence disease progression by regulating immune cell infiltration and controlling the burden or composition of microorganisms in the oral cavity, Previous researchers have indicated, significantly elevated salivary chemerin level in patients with periodontitis as compared to healthy controls30. Szydło et al. also demonstrated that an elevated level of salivary chemerin in periodontitis patients was associated with a higher degree of tissue destruction28,29. In the current study, we showed that chemerin present in the saliva of patients with periodontal disease as well as OSCC was significantly higher than in the saliva of healthy controls. Hence, chemerin’s ability to selectively destroy the oral bacteria may influence the onset and course of gum disease by altering the oral microbiome’s composition. Chemerin may also aid in the translation of microbial insult signals to a human physiological response by directing immune cells to infection sites. Chemerin activation in periodontal diseases may result in immunological deregulation. Chemerin inactivation, on the other hand, might be a method for preventing periodontitis-related inflammation and bone loss22.
The issue that we faced in our current study was that we were unable to determine the risk of OSCC among individuals with periodontitis since we included an equal number of patients with and without OSCC because it was a non-probability sampling approach and the study’s sample size was limited. Therefore, the results obtained are not generalizable. However, this is the first study in Pakistan to examine salivary chemerin levels among individuals with OSCC and periodontitis. This research suggests that salivary chemerin levels might be beneficial in identifying oral cancer at an early stage and developing disease-prevention methods. Further study is needed to determine the efficacy of this biomarker, and large, prospective, multicenter studies should be designed. Moreover, the outcome of the disease may be influenced by altering the factors that cause the disease to arise. Therefore, by putting an effort to prevent chronic periodontitis by maintaining good oral hygiene, we may not only reduce the incidence of various systemic diseases but also minimize the burden of oral cancer.
In this study salivary chemerin levels were higher in OSCC and chronic periodontitis group, compared to the healthy controls. Therefore, chemerin level in saliva might be considered a recommended diagnostic biomarker for early detection of OSCC in high-risk individuals.
The authors would like to thank the supervisor, co-supervisor and family for all the support.
The authors have no conflict of interest.
The ethics approval was obtained from the Ethics Review Committee of Ziauddin University, Karachi.
Informed consent was obtained from each participant of the study.
All authors equally contributed to this research study.
- Hema K, Smitha T, Sheethal H, Mirnalini SA. Epigenetics in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2017;21(2):252-259. doi: 10.4103/jomfp.JOMFP_150_17
- Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol. 2019;10:1-12. doi: 10.3389/fphys.2019.01476
- Awasthi N. Role of salivary biomarkers in early detection of oral squamous cell carcinoma. Indian J Pathol Microbiol. 2017;60(4):464-468. doi: 10.4103/IJPM.IJPM_140_16
- Ahmed R, Malik S, Khan MF, Khattak MR. Epidemiological and clinical correlates of oral squamous cell carcinoma in patients from north-west Pakistan. J Pak Med Assoc. 2019;69:1074-1078.
- Ye L, Jiang Y, Liu W, Tao H. Correlation between periodontal disease and oral cancer risk: a meta-analysis. J Cancer Res Ther. 2016;12(8):237-240. doi: 10.4103/0973-1482.200746
- Sahingur SE, Yeudall WA. Chemokine function in periodontal disease and oral cavity cancer. Front Immunol. 2015;6:1-15. doi: 10.3389/fimmu.2015.00214
- Gondivkar SM, Gondivkar RS, Gadbail AR, Chole R, Mankar M, Yuwanati M. Chronic periodontitis and the risk of head and neck squamous cell carcinoma: facts and figures. Exp Oncol. 2013;35(3):163-167.
- Özcan E, Saygun NI, Serdar MA, Kurt N. Evaluation of the salivary levels of visfatin, chemerin, and progranulin in periodontal inflammation. Clin Oral Invest. 2015;19(4):921-928. doi: 10.1007/s00784-014-1308-0
- Özcan E, Saygun NI, Ilıkçı R, Karslıoğlu Y, Muşabak U, Yeşillik S. Evaluation of chemerin and its receptors, ChemR23 and CCRL2, in gingival tissues with healthy and periodontitis. Odontology. 2018;106(1):29-36. doi: 10.1007/s10266-017-0297-2
- Goralski KB, Jackson AE, McKeown BT, Sinal CJ. More than an adipokine: the complex roles of chemerin signaling in cancer. Int J Mol Sci. 2019;20(19):4778. doi: 10.3390/ijms20194778
- Erdogan S, Yilmaz FM, Yazici O, Yozgat A, Sezer S, Ozdemir N, et al. Inflammation and chemerin in colorectal cancer. Tumor Biol. 2016;37(5):6337-6342.
- Wang C, Wu WK, Liu X, To KF, Chen GG, Yu J, Ng EK. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: a clinical and experimental study. Peptides. 2014;51:131-138. doi: 10.1016/j.peptides.2013.10.009
- Yamaguchi Y, Du XY, Zhao L, Morser J, Leung LL. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J Biol Chem. 2011;286(45):39510-39519. doi: 10.1074/jbc.M111.258921
- Wang N, Wang QJ, Feng YY, Shang W, Cai M. Overexpression of chemerin was associated with tumor angiogenesis and poor clinical outcome in squamous cell carcinoma of the oral tongue. Clin Oral Invest. 2014;18(3):997-1004. doi: 10.1007/s00784-013-1046-8
- Ghallab NA, Shaker OG. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin Oral Invest. 2017;21(3):937-947. doi: 10.1007/s00784-016-1846-8
- Eliot MN, Michaud DS, Langevin SM, McClean MD, Kelsey KT. Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer Causes Control. 2013;24(7):1315-1322. doi: 10.1007/s10552-013-0209-x
- Naseer R, Naz I, Mahmood MK. Frequency of delayed diagnosis of oral squamous cell carcinoma in Pakistan. Asian Pac J Cancer Prev. 2016;17(11):5037-5040. doi: 10.22034/APJCP.2016.17.11.5037
- Suresh GM, Koppad R, Prakash BV, Sabitha KS, Dhara PS. Prognostic indicators of oral squamous cell carcinoma. Ann Maxillofac Surg. 2019; 9(2): 364-370. doi: 10.4103/ams.ams_253_18
- Khurshid Z, Zafar MS, Khan RS, Najeeb S, Slowey PD, Rehman IU. Role of Salivary Biomarkers in Oral Cancer Detection. Adv Clin Chem. 2018;86:23-70. doi: 10.1016/bs.acc.2018.05.002
- Radhika T, Jeddy N, Nithya S, Muthumeenakshi RM. Salivary biomarkers in oral squamous cell carcinoma–An insight. J Oral Biol Craniofac Res. 2016;6(1):S51-S54. doi: 10.1016/j.jobcr.2016.07.003
- Cheng YS, Jordan L, Chen HS, Kang D, Oxford L, Plemons J, et al. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J Periodontal Res. 2017;52(3):428-437. doi: 10.1111/jre.12407
- Ghallab NA, Shaker OG. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin Oral Invest. 2017;21(3):937-947. doi: 10.1007/s00784-016-1846-8
- Patnaik K, Pradeep AR, Nagpal K, Karvekar S, Singh P, Raju A. Human chemerin correlation in gingival crevicular fluid and tear fluid as markers of inflammation in chronic periodontitis and type‐2 diabetes mellitus. J Investig Clin Dent. 2017;8(1):1-7. doi: 10.1111/jicd.12181
- Jose KP, Padma R, Abhraham BL. Chemerin: a potential marker of periodontal health. Int J Health Sci Res. 2016;6:143-145.
- Shin YJ, Choung HW, Lee JH, Rhyu IC, Kim HD. Association of periodontitis with oral cancer: a case-control study. J Dent Res. 2019;98(5):526-533. doi: 10.1177/00220345198275
- Perumalsamy S, Ain Aqilah Mohd Zin N, Teguh Widodo R, Azman Wan Ahmad W, Vethakkan RD, Zaman Huri H. Chemokine like receptor-1 (CMKLR-1) receptor: a potential therapeutic target in management of chemerin induced type 2 diabetes mellitus and cancer. Curr Pharm Design. 2017;23(25):3689-3698. doi: 10.2174/1381612823666170616081256
- Kumar JD, Holmberg C, Kandola S, Steele I, Hegyi P, Tiszlavicz L, et al. Increased expression of chemerin in squamous esophageal cancer myofibroblasts and role in recruitment of mesenchymal stromal cells. PLoS One. 2014;9(8):1-10. doi: 10.1371/journal.pone.0104877
- Szydło B, Kiczmer P, Świętochowska E, Ostrowska Z. Role of omentin and chemerin in metabolic syndrome and tumor diseases. Postepy Hig Med Dosw (Online). 2016;70:844-849. doi: 10.5604/17322693.1214137
- Lu Z, Liang J, He Q, Wan Q, Hou J, Lian K, Wang A. The serum biomarker chemerin promotes tumorigenesis and metastasis in oral squamous cell carcinoma. Clin Sci. 2019;133(5):681-695. doi: 10.1042/CS20181023
- Lee JY, Lee MK, Kim NK, Chu SH, Lee DC, Lee HS, et al. Serum chemerin levels are independently associated with quality of life in colorectal cancer survivors: A pilot study. PLoS One. 2017;12(5):1-12. doi: 10.1371/journal.pone.0176929
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
