By Sumaira Siddique Mughal1, Shaista Bakhat2, M Qamar ul Haq Noor3, Rida Sohail2, Yasmeen Taj2, Saba Sarwar1, Luqman Sutti1, Faisal Faheem4
- Department of Medical Microbiology, PNS SHIFA Hospital, Bahria University Health Sciences, Karachi, Pakistan.
- Department of Pathology, Bahria University Health Sciences, Karachi, Pakistan.
- PNS SHIFA Hospital, Karachi, Pakistan.
- Department of Physical Therapy, Bahria University Health Sciences, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD12-1/010
ORCID iD: 0000-0002-4180-0026
How to cite: Mughal SS, Bakhat S, Noor MQH, Sohail R, Taj Y, Sarwar S, et al. Fosfomycin Resistance in Clinical Isolates of Escherichia coli from Urinary Tract Infections in a Tertiary Care Hospital. Pak J Med Dent. 2023;12(1): 53-57. doi: 10.36283/PJMD12-1/010
Background: Fosfomycin is called an old-new antibiotic, because it was reintroduced for the treatment of urinary tract infections. The rampant use of antibiotics has led to Fosfomycin resistance reported from different parts of the world. The purpose of this study was to find out the frequency of Fosfomycin resistance in clinical isolates of Escherichia coli (E. coli) from urinary tract samples.
Methods: This cross-sectional study, including n=314 patients, was conducted in the Microbiology laboratory at Pakistan Naval Ship SHIFA Hospital, Pakistan from January to June 2022. Urine samples were inoculated on agar at 37±2°C for 24-48hrs. The growth of E. coli was confirmed by API 10S as per recommended Clinical Laboratory Standard Institute (CLSI) guidelines. Susceptibility testing was performed by the standard Kirby Bauer Disc Diffusion method. The Chi-square test was applied to categorical variables. p-value ≤ 0.05 was considered statistically significant.
Results: Out of 314 clinical isolates, 171 (54.5%) were females and 143 (45.5%) were males (mean age 49±10.3 (6-81) years). Sixty-six (21%) isolates were found resistant to Fosfomycin while 248 (79%) were sensitive. A significant difference was found between the gender (p=0.035), whereas, 29 (17.0%) females and 37 (25.9%) males were found resistant to Fosfomycin.
Conclusion: The resistance to Fosfomycin is increasing and it is an impending threat as oral treatment options are limited in urinary tract infections. However, improved surveillance may help in controlling nosocomial infection along with the rational use of antibiotics can prevent and reduce its spread.
Keywords: Fosfomycin; Culture; Susceptibility; Urinary Tract Infections (UTIs).
Urinary tract infections (UTIs) are the most widespread community and hospital-acquired infections, which need certain treatment measures1. Gram-negative rods like E. coli are the leading reported pathogen involved in UTIs 2. There has been a substantial rise in multidrug-resistant Escherichia coli strains including those producing β-lactamases and carbapenemases in the last decade, leading to limiting therapeutic options in hand to treat urinary tract infections and UTIs 3. WHO GLASS (Global Antimicrobial Resistance and Use Surveillance System) report of 2017-2018 points out resistance of >70% towards ceftriaxone and ciprofloxacin in E. coli isolated from urinary tract infection in Pakistan4. In Pakistan resistance to Fosfomycin is low as there was only one reported case in the Bullens et al. study in an isolate of E. coli5. Resistance to Fosfomycin from other parts of the world is reported as 8.3% in Italy, 4.6% in Spain, 2.9% in the UK (United Kingdom), and 1.9% from Belgium and Russia6.
Fosfomycin was first discovered in Spain in 1969 and was commenced in Europe in a period of 1970s 7. Fosfomycin is a bactericidal antibiotic, which is suitable to treat diseases by MDR (multi-drug resistant) pathogens. Its mode of action is by blocking the transferase enzyme UDP-N-acetylglucosamine enolpyruvyl (MurA), which is involved in stopping the production of murein peptidoglycan. Peptidoglycan biosynthesis is also an important regulator of bacterial cell division8. Mechanisms of Fosfomycin resistance are mediated towards E. coli by the ability of a pathogen to reduce the uptake of the drug and elaborate enzymes are inactivated and alter the target receptors of the drug, thus rendering its effectiveness9. Among the mechanism, the reduced drug uptake occurs most frequently mediated by a mutation in chromosomes like point mutations, base insertions, and base deletions which alter the transport mechanism of the drug reducing its uptake10.
Enzymes that modify the configuration of Fosfomycin are mediated by plasmids carrying genes (fosA-like genes with K and Mn2+-dependent glutathione S-transferase; fosB-like genes Mg2+-dependent thiol S-transferase and; fosX with Mn2+-dependent epoxide hydrolase) 11. Rarely resistance towards Fosfomycin can be carried out by modifications of enzyme MurA. Two cases of this kind of resistant isolates were detected in Japan while global expression of MurA is not a phenomenon commonly reported so far in E. coli 12,13. In uncomplicated UTIs, this drug is recommended and can be used as a 3-grams single dose therapy against E. coli and Enterococcus faecalis. Its activity against both Gram-negative and Gram-positive bacteria is fairly good with little cross-resistance with other antibiotics. Different cases of Fosfomycin resistance from different parts of the world were reported, as a result of the rampant use of antibiotics. Therefore, this study aimed to know the frequency of Fosfomycin resistance in E. coli isolated from suspected UTI patients in a tertiary care hospital.
This was a descriptive cross-sectional study conducted in the Microbiology laboratory at Naval Ship SHIFA Hospital, Pakistan from January 2022 to July 2022. All the urine samples with pus cells more than 4-5/HPF under the microscope were included in the study. Both genders, outdoor patients and in-door patients were included in the study. Repeat specimens, with broken or damaged containers, and specimens with contaminated and mixed growth were excluded from the study. Patients were informed about the study and took written consent. The ethical study was approved ethical review board of PNS Shifa hospital.
In the protocol, all the urine samples were inoculated on cysteine lactose electrolyte deficient (CLED) agar (Oxoid, UK) by strip method for obtaining a semi-quantitative bacterial count. Inoculated plates were incubated for 24-48 hours at 35°C–37°C in ambient air. The growth of E. coli was confirmed by using API (analytical profile index) 10S (bioMérieux, France). A suspension of the organism to be tested was matched with a turbidity of a 0.5 McFarland standard 1 × 108 CFU (colony forming unit)/ml). Antibiotic susceptibility testing of E. coli towards Fosfomycin was done on Muller-Hinton Agar (Oxoid, UK) by applying the modified Kirby-Bauer disk diffusion method which is the recommended method by CLSI (Clinical Laboratory Standard Institute) guidelines14,15. Fosfomycin resistance was noted by using a Fosfomycin disc 50 μg (Oxoid, UK). E. coli ATCC 25922 was used as the control strain. CLSI breakpoints for E. coli were used that are more than or equal to 15mm susceptible and less than 15mm resistant.
Statistical analysis was done by SPSS version 25. Mean and Standard deviation was calculated for variables like age and gender. Frequency and percentages were calculated for all categorical variables. Independent sample t-test was applied between age and Fosfomycin-resistant E. coli. The Chi-square test was applied to see the significance between two categorical variables. p-value ≤ 0.05 is considered to be statistically significant.
A total of 314 isolates of E. coli were isolated, according to the Open Epi calculator and included in the study. Out of 314 clinical isolates, 171 (54.5%) were from females and 143 (45.5%) were from the male. Outdoor patients were included in the study more than hospitalized. Fosfomycin resistance was noticed in 21% patients as shown in Table 1.
Table 1: Frequency of Fosfomycin resistance concerning to gender, inpatient/outpatient data.
| Variables | Frequency n (%) | |
| Gender (n=314) | Female | Male |
| 171(54.5) | 143(45.5) | |
| Inpatient/Outpatient | Indoor | Outdoor |
| 91(29.0) | 223(71.0) | |
| Fosfomycin Resistant E. coli | Resistant | Sensitive |
| 66(21.0) | 248(79.0) | |
The mean age of the patients was 49± 10.3 (6-81) years. Out of a total of 314 clinical isolates, 29 (17.0%) in females, 37 (25.9%) in males were found resistant and 248 (79%) were sensitive to Fosfomycin (Table 2). A significant difference was found between the gender (p=0.035). Out of 314 isolates, 223 isolates were from outdoor patients while 91 were from indoor patients (Table 2). The outdoor patients were found more sensitive 179(80.3%) to Fosfomycin than indoor patients 69 (75.8%) p=0.445). The resistance and sensitivity of bacteria to Fosfomycin are shown in Figure 1.
Table 2: Relationship of Fosfomycin with gender and indoor /outdoor patients.
| Fosfomycin | Gender
Frequency n (%) |
Total
Frequency n (%) |
p-Value | Setting
Frequency n (%) |
Total
Frequency n (%) |
p-Value | ||
| Female | Male | Outdoor | Indoor | |||||
| Resistant | 29 (17.0%) | 37 (25.9%) | 66 (21.0%) | 0.035 | 44 (19.7%) | 22 (24.2%) | 66 (21%) | 0.445 |
| Sensitive | 142 (83%) | 106 (74.1%) | 248 (79%) | 179(80.3%) | 69 (75.8%) | 248 (79%) | ||
| Total | 171 (100%) | 143(100%) | 314(100%) | 223(100%) | 91 (100%) | 314 (100%) | ||
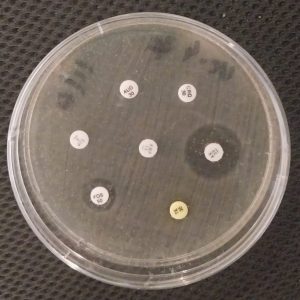
Figure 1: Culture medium showed Fosfomycin resistance.
In this study 314 cases of UTI, were studied for sensitivity and resistance pattern of E. coli isolates against Fosfomycin and the result showed 21% resistance while 79% were sensitive. Male and outdoor patients outnumbered females in exhibiting symptoms of urinary tract infection and resistance. The growing resistance of pathogens towards multiple antibiotics limiting therapeutic options has created panic among the health care communities16.
Among different common infections, urinary tract infections are considered the most common infections. Fosfomycin an old drug has very good activity in uncomplicated UTIs. Nitrofurantoin and Fosfomycin are recommended for first-line remedies because of the sensitivity of E. coli to both these agents. There is less clinical, epidemiological, or molecular data on E. coli resistant to Fosfomycin detected from UTIs. Unfortunately, resistance to this drug is also increasing and reports of resistance from various parts of the world have been reported17,18.
Fosfomycin is the antibiotic of choice for treating pathogens with resistance to main-line antibiotics (MDR) and extensively drug-resistant (XDR) infections. Therefore, rising resistance towards this drug drastically diminishes accessible therapy options. According to our study, urinary tract infections were more common in females (54.5%) compared to males (45%) which is in association with another study done by Ganesh et al. in 2019 19. This fact is collaborated by another study done by Medina et al. in 2019 as well20. This is also detected by a study conducted in Singapore. Their study revealed that urinary tract infection is the most common infection among females, especially from the community21. This can be endorsed to the truth that females have a short urethra and decreased level of estrogen. Lower levels of estrogen lead to a reduction of normal vaginal flors22. We observed that outdoor patients are more prone to get UTIs than indoor patients, this fact collaborates with another study by Khan et al. 201423. The community-acquired urinary tract is common compared to hospitalized, also following a study in Poland24. This is a reflection of illiteracy, low socioeconomic status and poor hygiene. Our study detected 21% Fosfomycin-resistant cases in E. coli isolates from urinary samples, this figure is in close association with a study conducted by Michalopoulos et al. in 2022 25. Similar results were observed in a study in Israel that urinary tract infections are frequently detected in the community and Fosfomycin resistance is around 23% 26.
Fosfomycin resistance (7.8%) is also reported in a study by Li et al. in 2015, in China., although this antimicrobial agent is not commonly prescribed in China 27 as shown in Table 3. However, delineated Fosfomycin resistance (14.1%) in isolates of E. coli in 2020 was found in association with our study28. A study was conducted at Rawalpindi and Islamabad, which detected resistance of 3% which was lower than the current study. Therefore, this indicated that it is difficult to use Fosfomycin as empirical therapy29. The escalating resistance of Fosfomycin has become a serious public-health trepidation as it limits the alternative antimicrobial agents available for therapy. A study from, Czech Republic reported the very high susceptibility to Fosfomycin trometamol of urinary tract infection pathogens, particularly Gram-negative rods including those producing β-lactamase30 as shown in Table 3.
| Population | Resistance (%) | Sensitivity (%) | Studies |
| Pakistani (Karachi) | 21% | 79% | Current Study |
| Israeli | 23% | 77% | Peretz et al.26 |
| Chinses | 7.8% | 92.2% | Li et al.27 |
| Pakistani (Rawalpindi and Islamabad) | 3% | 97% |
Malik et al.29
|
| Czech Republic | 4.2% | 95.8% | Fajfr et al.30 |
Table 3: Fosfomycin resistance and sensitivity rates among different populations.
The limitations of this study were a single-centered study with a small sample size. Due to time and constriction of finance, the tested strain was only detected on basis of biochemical identification using API 20E. Molecular identification on basis of resistant genes like mcr-1, mrgB, or other mutations was not detected. Hence further studies are required to identify genetic mutations related to Fosfomycin resistance.
The study found that Fosfomycin resistance to the most prevalent infection, E. coli, is rising in our setup of Pakistan. This highlights the significance of strict infection control measures, strict guidelines on the use of this drug empirically and promoting culture and sensitivity in all the cases of suspected UTIs. These strategies will limit the use of this precious drug and decrease resistance. Furthermore, more studies are required at the multicenter level with a larger sample size to know the exact Fosfomycin resistance
The authors would like to acknowledge hospital staff and doctors for their immense contribution.
The authors declared no conflict of interest.
This study was approved by the Ethics Review Committee of PNS Shifa Hospital.
Written consent was signed by concerned patients.
All authors equally contributed to this research write-up.
- Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother. 2018;50(1):67-100. doi: 10.3947/ic.2018.50.1.67
- Gad AH. The Occurrence of Multidrug Resistant coli which produce ESBL and cause urinary tract infections. J Appl Microbiol. 2017;1(2):1-8. doi: 10.21767/2576-1412.100008
- Arana DM, Rubio M, Alós JI. Evolution of antibiotic multiresistance in Escherichia coli and Klebsiella pneumoniae isolates from urinary tract infections: a 12-year analysis (2003–2014). Enferm Infecc Microbiol Clin. 2017;35(5):293-298. doi: 10.1016/j.eimc.2016.02.018
- World Health Organization Global Antimicrobial Resistance Surveillance System (GLASS) report, early implementation 2017–2018.Geneva, Switzerland: WHO; 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/279656/9789241515061-eng.pdf
- Bullens M, de Cerqueira Melo A, Raziq S, Lee J, Khalid GG, Khan SN, et al. Antibiotic resistance in patients with urinary tract infections in Pakistan. Public Health Action. 2022;12(1):48-52. doi: 10.5588/pha.21.0071
- Tutone M, Johansen TE, Cai T, Mushtaq S, Livermore DM. SUsceptibility and Resistance to Fosfomycin and other antimicrobial agents among pathogens causing lower urinary tract infections: findings of the SURF study. Int J Antimicrob Agents. 2022;59(5):1-7. doi: 10.1016/j.ijantimicag.2022.106574
- Hendlin D, Stapley EO, Jackson M, Wallick H, Miller AK, Wolf FJ, et al. Phosphonomycin, a new antibiotic produced by strains of Streptomyces. Science. 1969;166(3901):122-123. doi: 10.1126/science.166.3901.122
- Falagas ME, Athanasaki F, Voulgaris GL, Triarides NA, Vardakas KZ. Resistance to fosfomycin: mechanisms, frequency and clinical consequences. Int J Antimicrob Agents. 2019;53(1):22-28. doi: 10.1016/j.ijantimicag.2018.09.013
- Lucas AE, Ito R, Mustapha MM, McElheny CL, Mettus RT, Bowler SL, et al. Frequency and mechanisms of spontaneous fosfomycin nonsusceptibility observed upon disk diffusion testing of Escherichia coli. J Clin Microbiol. 2018;56(1): e01368-17. doi: 10.1128/JCM.01368-17
- Martín-Gutiérrez G, Docobo-Pérez F, Rodriguez-Beltrán J, Rodríguez-Martínez JM, Aznar J, Pascual A, et al. Urinary tract conditions affect fosfomycin activity against Escherichia coli strains harboring chromosomal mutations involved in fosfomycin uptake. Antimicrob Agents Chemother. 2018;62(1): e01899-17. doi: 10.1128/AAC.01899-17
- Yang TY, Lu PL, Tseng SP. Update on fosfomycin-modified genes in Enterobacteriaceae. J Microbiol Immunol Infect. 2019;52(1):9-21. doi: 10.1016/j.jmii.2017.10.006
- Silver LL. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med. 2017;7(2):1-12. doi: 10.1101/cshperspect.a025262
- Takahata S, Ida T, Hiraishi T, Sakakibara S, Maebashi K, Terada S, et al. Molecular mechanisms of fosfomycin resistance in clinical isolates of Escherichia coli. Int J Antimicrob Agents. 2010;35(4):333-337. doi: 10.1016/j.ijantimicag.2009.11.011
- Siddiqui GM, Hannan A. Comparison of standard Kirby-Bauer and modified Kirby-Bauer disk diffusion methods for the detection of methicillin resistance among Staphylococcus. Pak J Pathol. 1996;7(1):37-41.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for Antimicrobial susceptibility testing. Approved Guideline. 2022;34(1):M100–Ed32. Available from: https://clsi.org/standards/products/microbiology/documents/m100/
- Bilal H, Khan MN, Rehman T, Hameed MF, Yang X. Antibiotic resistance in Pakistan: a systematic review of past decade. BMC Infect Dis. 2021;21(1):1-9. doi: 10.1186/s12879-021-05906-1
- Ganesh R, Shrestha D, Bhattachan B, Rai G. Epidemiology of urinary tract infection and antimicrobial resistance in a pediatric hospital in Nepal. BMC Infect Dis. 2019;19(1):1-5. doi: 10.1186/s12879-019-3997-0
- Qamar S, Shaheen N, Shakoor S, Farooqi J, Jabeen K, Hasan R. Frequency of colistin and fosfomycin resistance in carbapenem-resistant Enterobacteriaceae from a tertiary care hospital in Karachi. Infect Drug Resist. 2017; 10: 231-236. doi: 10.2147/IDR.S136777
- Al-Badr A, Al-Shaikh G. Recurrent urinary tract infections management in women: a review. Sultan Qaboos Univ Med J. 2013; 13(3): 359-367. doi: 10.12816/0003256
- Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:3-7. doi: 10.1177/175628721983217
- Ng QX, Peters C, Venkatanarayanan N, Goh YY, Ho CY, Yeo WS. Use of Lactobacillus spp. to prevent recurrent urinary tract infections in females. Med Hypotheses. 2018;114:49-54. doi: 10.1016/j.mehy.2018.03.001
- Stapleton AE. The vaginal microbiota and urinary tract infection. Book Chapter 5: Urinary Tract Infections: Molecular Pathogenesis and Clinical Management. 2017:79-86. doi: 10.1128/9781555817404.ch5
- Khan IU, Mirza IA, Ikram A, Afzal A, Ali S, Hussain A, et al. Antimicrobial susceptibility pattern of bacteria isolated from patients with urinary tract infection. J Coll Physicians Surg Pak. 2014;24(11):840-844.
- Kot B. Antibiotic Resistance Among Uropathogenic. Pol J Microbiol. 2019 ;68(4):403-415. doi: 10.33073/pjm-2019-048
- Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis. 2011;15(11):e732-e739. doi: 10.1016/j.ijid.2011.07.007
- Peretz A, Naamneh B, Tkhawkho L, Nitzan O. High rates of fosfomycin resistance in Gram-negative urinary isolates from Israel. Microb Drug Resist. 2019;25(3):408-412. doi: 10.1089/mdr.2018.0393
- Li Y, Zheng B, Li Y, Zhu S, Xue F, Liu J. Antimicrobial susceptibility and molecular mechanisms of fosfomycin resistance in clinical Escherichia coli isolates in mainland China. PLoS One. 2015;10(8):1-7. doi: 10.1371/journal.pone.0135269
- Loras C, Mendes AC, Peixe L, Novais Â, Alós JI. Escherichia coli resistant to fosfomycin from urinary tract infections: Detection of the fosA3 gene in Spain. J Glob Antimicrob Resist. 2020;21:414-416. doi: 10.1016/j.jgar.2020.01.023
- Malik J, Javed N, Malik F, Ishaq U, Ahmed Z. Microbial resistance in urinary tract infections. Cureus. 2020;12(5):1-5. doi:10.7759/cureus.8110
- Fajfr M, Louda M, Paterová P, Ryšková L, Pacovský J, Košina J, et al. The susceptibility to fosfomycin of Gram-negative bacteria isolates from urinary tract infection in the Czech Republic: data from a unicentric study. BMC Urol. 2017;17(1):1-6. doi: 10.1186/s12894-017-0222-6
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
