By Syed Mehmood Hasan1, Sadaf Razzak1, Asma Shabbir1, Syed Muhammad Hasan2, Masood Husain3
- Department of Pathology, Sind Medical College, Jinnah Sindh Medical University (JSMU), Karachi, Pakistan.
- Department of Medicine, Dow University of Health Sciences, Karachi, Pakistan.
- Pathology Section, Tahir Laboratory, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD12-1/005
How to cite: Hasan SM, Razzak S, Shabbir A, Hasan SM, Husain M. Extensively Drug-Resistant Typhoid: Antibiotic Susceptibility Pattern of an Emerging Menace in Karachi. Pak J Med Dent. 2023;12(1): 18-23. doi: 10.36283/PJMD12-1/005
Background: Salmonella typhi, a rod-shaped Gram-negative bacterium that stands responsible for causing typhoid fever, in places with poor sanitation and polluted water. Injudicious antibiotic practices have significantly added to the rise of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Salmonella typhi. This study aimed to determine the frequency and antibiotic susceptibility pattern of Salmonella typhi in blood cultures of patients with fever.
Methods: This cross-sectional retrospective study was conducted from November 2018 to November 2019 at Tahir Laboratory, Karachi. All clinically suspected typhoid fever cases were cultured and Salmonella typhi was identified by traditional methods using standard microbiological procedures. Antibiotic susceptibility testing was done by the Kirby-Bauer disk diffusion method and a combined disc test was done to detect Extended Spectrum β-Lactamase production in XDR cases.
Results: Out of 394 samples received for blood culture, 99(25.12%) were gram-positive, 158(40.10%) were gram-negative and 137 (34.7%) showed no growth. In 158 gram-negative samples, 92(58.22%) were of Salmonella typhi, consisting of 40(43.47%) cases of children aged 0-10 years. MDR and XDR Salmonella typhi isolates were 25(27.2%) and 8(8.7%) respectively, showing a male preponderance of 20 (60.60%). Both the MDR and XDR strains were sensitive to Meropenem and Azithromycin. Extended Spectrum β-Lactamase production was confirmed by combined disc test in all the XDR cases.
Conclusion: The emergence of multidrug-resistant and extensively drug-resistant Salmonella typhi requires surveillance to see antibiogram trends for empirical therapy and to facilitate the prevention of the spread of the disease.
Keywords: Salmonella typhi; Antibiotic Susceptibility Test; Extensively Drug-Resistant; Multidrug-Resistant.
Salmonella typhi is a rod-shaped Gram-negative bacte- rium of the family Enterobacteriaceae responsible for causing typhoid fever1. This infection is common in places that have poor mechanisms of sanitation and people drink unsafe drinking water2. Treatment modali- ties have changed for this microorganism due to its continuous and changing resistance pattern. Nearly a decade back, according to a report by the WHO, the incidence of typhoid fever was approximately 21 million cases and typhoid-related complications, especially intestinal perforation were at 3%3. The annual incidence of cases in our neighboring countries like China is 29.3 cases per 100,000 persons per year and in India, it is 493.5/1000004. In Pakistan, the incidence is comparable to in India which is high considering the differences in the area between the two countries5. In 2018, the frequency of multi-drug resistant (MDR) Salmonella typhi was 21% 6. Initially, chloramphenicol has used as an antibiotic of choice but due to the resistance, cell wall inhibitors and protein synthesis inhibitors like ampicillin and ciprofloxacin respectively were used7. In the past few years, the worry- ing aspect of typhoid fever is that since the year 2016, it has developed a resistance to many antibiotics such as the emergence and spread of multidrug-resistant (MDR) Salmonella typhi and extensively-drug resistant (XDR) Salmonella typhi throughout Pakistan has put many clinicians and microbiologists in a complex situation8.
The definition of Multidrug resistance (MDR) typhoid is resistance to the first-line antibiotics ampicillin, cotrimoxazole, and chloramphenicol8. Not only MDR but now XDR typhoid is also reported in Pakistan. Extensive drug resistance (XDR) typhoid is defined as MDR plus resistance to fluoroquinolones and cephalosporins9. MDR strains of this microorgan- ism are unaffected by chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole, which are traditional first-line antibiotic drugs and have sporadic resistance against second-line drugs, such as fluoroquinolones. An extensive drug-resistant (XDR) Salmonella typhi strain has emerged that is resistant to all recommended antibiotics for typhoid fever, including first-line and second-line drugs as well as third-generation cephalosporins. It is alarm- ing to see data from a private laboratory telling us of the spread of the new XDR strain which requires immediate action10. The XDR strain is sensitive to a handful of drugs such as azithromycin and mero- penem and overuse of these antibiotics can lead to untreatable strains10. Although the global spread of XDR Salmonella typhi showing detection of extend- ed-spectrum β-lactamase has been proven in several studies, information regarding the spread in our country is still insufficient and should be evaluat- ed through single center studies too11.The study aimed to assess the frequency and current status of antibiotic susceptibility of Salmonella typhi in blood cultures of patients with a history of fever.
This cross-sectional retrospective study was conducted from the period of November 2018 to November 2019 at Tahir Laboratory, Karachi. The data of confirmed cases of Salmonella typhi in blood culture reports of the patients suspected clinically of typhoid fever was taken. The isolates were cultured and identified by traditional methods using standard microbiological procedures. Isolates were confirmed as Salmonella typhi by serological reactions to O, H, and Vi antigens.
The antibiotic susceptibility testing was done by the Kirby-Bauer disk diffusion method and was used to identify the antibiotic susceptibility test results for ampicillin, co-trimoxazole, chloramphenicol, ciprofloxacin, cefixime, ceftriaxone, fosfomycin, meropenem and azithromycin. The frequency of MDR and XDR Salmonella typhi was studied and sensitivity to meropenem and azithromycin was calculated and analyzed by using Statistical Package for Social Sciences (SPSS) version 21 and appropriate descriptive statistical tests including frequency, mean and standard deviation was applied. The association of MDR and XDR with age and gender was calculated by the Chi-square test. p-value < 0.05 was considered significant. A combined disc diffusion test confirmed ESBL production in XDR strains.
Out of the total 394 samples received for blood culture, 99(25.12%) were gram-positive, 158(40.10%) were gram-negative and the remaining showed no growth. Out of 158 gram-negative samples, 92(58.22%) were of Salmonella typhi. As shown in Table 1, there is male predominance and a younger age group seems to be more infected by the resistant strains.
Table 1: Distribution of multi-drug resistance (MDR) and extensive-drug resistance (XDR) Salmonella typhi infection in different variables.
| Variables | MDR (n=25)
Frequency n (%) |
p-Value | XDR (n=8)
Frequency n (%) |
p-Value | Salmonella typhi (Infection
Age-wise data) |
| Gender | |||||
| Male | 15 (60%) | 0.97 | 5 (62.5%) | 0.87 | |
| Female | 10 (40%) | 3 (37.5%) | |||
| Age | |||||
| Children 0-10 years | 12 (48%) | 0.800 | 2 (25%) | 0.227 | 40 (43.5%) |
| Adolescent 11-19years | 7 (28%) | 4 (50%) | 28 (30.4%) | ||
| Adults 20-59 years | 4 (16%) | 1 (12.5%) | 19 (20.7%) | ||
| Seniors 60 years and above | 2 (8%) | 1 (12.5%) | 5 (5.4%) | ||
When the antibiotic susceptibility test of these isolates was done, it revealed that all the isolates of Salmonella typhi were sensitive to meropenem and azithromycin. The highest resistance was seen against amoxicillin/clavulanic acids (89.13%) followed by cefixime (71.73%) and ceftriaxone (63.04%) respectively as shown in Table 2.
Table 2: Antibiotics sensitivity and resistance pattern among Salmonella typhi isolates.
| Antibiotics | Sensitivity (%) | Intermediate (%) | Resistance (%) |
| Meropenem | 100% | 0 | 0 |
| Azithromycin | 100% | 0 | 0 |
| Amoxicillin/ Clavulanic acid | 10% | 0.87% | 89.13% |
| Cefixime | 28.27% | 0 | 71.73% |
| Ceftriaxone | 35.96% | 1% | 63.04% |
MDR isolates were mainly resistant to three antibiotics: ampicillin, chloramphenicol and co-trimoxazole. Out of 92 Salmonella typhi, 25(27.2%) were MDR isolates and XDR isolates were 8(8.7%) (Figure 1). Extended Spectrum β-Lactamase production was confirmed by combined disc test in all XDR cases as shown in Figure 2.
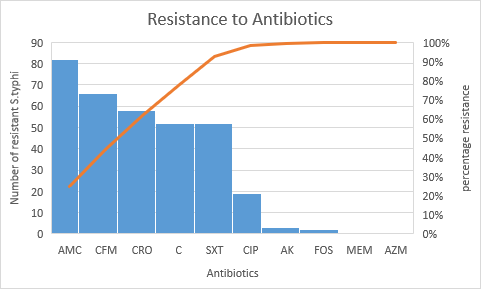
Figure 1: Resistance pattern of extensive-drug resistance and multi-drug resistance of Salmonella typhi isolates.
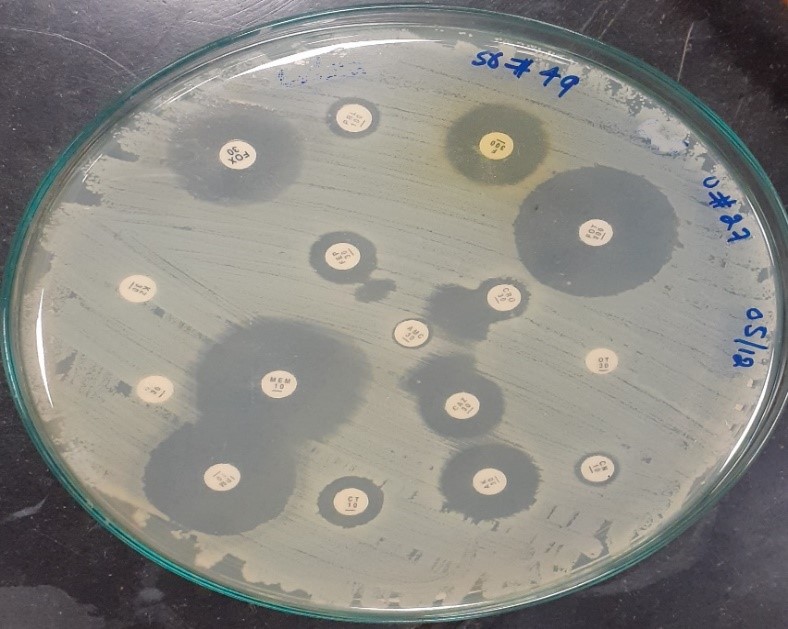
Figure 2: Disc diffusion test confirming extended-spectrum beta-lactamases (ESBL) production in MDR and XDR Salmonella typhi strains.
In a developing country like Pakistan, the most frequent infectious disease presenting with complications in a medical and surgical ward is typhoid fever12. Multiple factors are responsible for its spread, the most common is consuming contaminated water supplies, improper sanitary exercises, and limited antimicrobial susceptibility data13. Morbidity and mortality cases of infections by Salmonella typhi have been reported from Asia14. This retrospective study was done in a private laboratory in Karachi to see the antimicrobial sensitivity pattern for Salmonella enterica serovar Typhi infection in blood culture samples and it was seen that 43.5% of patients were of age group 10 or below followed by adolescents (30.4%). Since 27.17% of the isolates were MDR and XDR isolates to be 8.69% which showed additional resistance to cephalosporins and fluoroquinolones. Both the MDR and XDR strains were present more in males and also in the age group of 0-10 years. Regarding gender, more males were affected by this infection which is also supported by studies done in Bangladesh and China15,16. Because women stayed home due to several reasons and their male counterparts are outside due to work may more likely to ingest unhygienic food from different vendors but this needs to be explored17.
Considering treatment with ciprofloxacin as the second option for patients with typhoid fever, it leaves little option as our research shows 20.65% of the microorganism resistant to it and the possibility of intermediate resistance as seen in other countries, leaving very few options in patients with MDR Salmonella typhi infection18. Ceftriaxone is the drug of choice for the treatment of typhoid fever in South Asian countries and is generally used for empirical therapy which is likely to drive cephalosporin resistance among Salmonella typhi and many other Gram-negative bacteria19. In our case, ceftriaxone resistance is 63.04% which becomes a part of a small number of studies reported that showed resistance to cephalosporins, especially in other cities of Pakistan20. One of the important factors that make Gram-negative microorganisms resistant to antibiotics is producing β-lactamases enzyme. Many cases of Salmonella typhi resistant to β-lactamase by ESBL production have been reported in nearby Asian countries21,22. The XDR isolates in our present study, which were 8.7% of the total cases, showed ESBL test positive and fully susceptible to azithromycin and meropenem. Progressing to the more common use of azithromycin and meropenem will result in developing resistance against these antibiotics in near future, for the treatment of these XDR strains will accelerate the rise of azithromycin-resistant strains as well in the coming days. The XDR cases from Sindh especially Karachi, Pakistan highlight the rise of extensive cephalosporins resistance among Salmonella typhi strains, which demands the implication of a vital action plan before such strains become the usual phenotypes that will ultimately lead to treatment failure for typhoid fever with the available antimicrobial agents23.
Improper antibiotic prescription practices have significantly summed up in developing resistance to multiple antibiotics24. The emergence of multidrug-resistant and extensively drug-resistant Salmonella typhi demands ongoing surveillance to type antibiogram trends for empirical therapy and stop spread25. Looking at the results of this study, it seems inevitable that soon the treatment given to patients for Salmonella typhi infections will be difficult and few options will be available to counter this disease.
MDR strains of Salmonella typhi resistant to ampicillin, chloramphenicol and trimethoprim have been exposed in the outbreaks in South and Southeast Asia as well as in Africa26. As the flare-up of XDR Salmonella typhi was reported initially in Hyderabad, Sindh in 2016 27, this upsurge further spread to other parts of the province and Karachi, which is the largest city of the country, was vastly affected. Due to this, travel history to Pakistan became important in other countries where MDR and XDR Salmonella typhi was detected28. Minimum inhibitory concentrations of antibiotics in all isolates were not determined which contributed to the limitation of this study. Furthermore, data from multiple centers in the same area was not taken, which could not identify the brunt of disease in that area.
Apart from the fact that safe drinking water, hygiene and uncontaminated food items play a major role in preventing this disease, the role of vaccines cannot be ignored. But once the infection is developed, antibiotics are the mainstay for the treatment of Salmonella typhi infection.
The authors would like to recognize the assistance that they received from Miss Lubna Razzak for assisting in this article.
There was no conflict of interest among the authors.
Consent taken by all patients and parents of children enrolled in the study.
All authors equally contributed to this research study.
- Shah SR. Salmonella typhi as a Pathogenic Organism—-A Mini Review. Pak-Euro J Med Life Sci. 2021;4(Special Is):S105-S10. doi: 10.31580/pjmls.v4iSpecial Is.1671
- Qamar FN, Yousafzai MT, Khalid M, Kazi AM, Lohana H, Karim S, et al. Outbreak investigation of ceftriaxone-resistant Salmonella enterica serotype Typhi and its risk factors among the general population in Hyderabad, Pakistan: a matched case-control study. Lancet Infect Dis. 2018;18(12):1368-1376. doi: 10.1016/S1473-3099(18)30483-3
- Agbons BO. An eHealth system for monitoring the relapse of Salmonella typhi in outpatients. J Med Internet Res. 2020. doi: 10.2196/18658. Online ahead of print.
- Das JK, Hasan R, Zafar A, Ahmed I, Ikram A, Nizamuddin S, et al. Trends, associations, and antimicrobial resistance of Salmonella typhi and Paratyphi in Pakistan. Am J Trop Med Hyg. 2018; 99(3 Suppl): 48-54. doi: 10.4269/ajtmh.18-0145
- Afshan H, Jamil S, Fatima S. Extensively drug resistant typhoid fever in pakistan–analysis of current situation. RADS J Pharm Pharm Sci. 2019;7(4):227-231.
- Fatima G, Kazmi SS, Kainat S. XDR/MDR Salmonella: An experience from a tertiary care hospital, Karachi, Pakistan. Int J Infect Dis. 2020;101:37. doi: 10.1016/j.ijid.2020.09.131
- Patil N, Mule P. Sensitivity pattern of Salmonella typhi and Paratyphi a isolates to chloramphenicol and other anti-typhoid drugs: An in vitro Infect Drug Resist. 2019; 12: 3217-3225. doi: 10.2147/IDR.S204618
- Hussain A, Satti L, Hanif F, Zehra NM, Nadeem S, Bangash TM, et al. Typhoidal Salmonella strains in Pakistan: an impending threat of extensively drug-resistant Salmonella typhi. Eur J Clin Microbiol Infect Dis. 2019;38(11):2145-2149. doi: 10.1007/s10096-019-03658-0
- Shahid S, Mahesar M, Ghouri N, Noreen S. A review of clinical profile, complications and antibiotic susceptibility pattern of extensively drug-resistant (XDR) Salmonella typhi isolates in children in Karachi. BMC Infect Dis. 2021;21(1):1-9. doi: 10.1186/s12879-021-06599-2
- Saeed N, Usman M, Khan EA. An overview of extensively drug-resistant Salmonella typhi from a tertiary care hospital in Pakistan. Cureus. 2019;11(9):1-6. doi:10.7759/cureus.5663
- Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. MBio. 2018;9(1):1-10. doi: 10.1128/mBio.00105-18
- Fida S, Mansoor H, Saif S, Iqbal J, Khan AQ. Clinical Perspectives of multiple and extensively drug-resistant typhoid; result from a tertiary care hospital from Pakistan. J Infect Dev Ctries. 2021;15(04):530-537. doi:10.3855/jidc.13539
- Matrajt G, Lillis L, Meschke JS. Review of methods suitable for environmental surveillance of Salmonella typhi and Paratyphi. Clinical Infectious Diseases. 2020;71(Supplement_2):S79-S83. doi: 10.1093/cid/ciaa487
- Marchello CS, Birkhold M, Crump JA. Complications and mortality of typhoid fever: A global systematic review and meta-analysis. J Infect. 2020;81(6):902-910. doi: 10.1016/j.jinf.2020.10.030
- Ahmed Z, Rahman T. An epidemiological and prevalence study of Salmonella typhi and Salmonella paratyphi with antibiotic sensitivity pattern of different age group patients in Dhaka city, Bangladesh. Int J Sci Eng Res. 2021;12(11): 1124-1129.
- Wen-Juan W, Xiao-Li X, Jun-Ying Z, Lin D, Li-Hong S, Li-Jing X, et al. Epidemiological Investigation of Salmonella enterica Isolates in children with diarrhea in Chengdu, China. Jundishapur J Microbiol. 2021; 14(8):1-7. doi: 10.5812/jjm.119034
- Ngolo LJ, Fur NJ, Olugbenga OO. Extended beta-lactamase (ESBL) producing Salmonella typhi from presumptive typhoid patients in Nasarawa state, Nigeria. 2018. Greener J Epidemiol Public Health. 2018; 6(3): 80-86. doi: 10.15580/GJEPH.2018.3.050918069
- François Watkins LK, Winstead A, Appiah GD, Friedman CR, Medalla F, Hughes MJ, et al. Update on extensively drug-resistant Salmonella serotype Typhi infections among travelers to or from Pakistan and report of ceftriaxone-resistant Salmonella serotype Typhi infections among travelers to Iraq – United States, 2018-2019. MMWR Morb Mortal Wkly Rep. 2020;69(20):618-622. doi: 10.15585/mmwr.mm6920a2
- Yousafzai MT, Qamar FN, Shakoor S, Saleem K, Lohana H, Karim S, et al. Ceftriaxone-resistant Salmonella typhi outbreak in Hyderabad city of Sindh, Pakistan: high time for the introduction of typhoid conjugate vaccine. Clini Infect Dis. 2019;68(Supplement_1):S16-S21. doi: 10.1093/cid/ciy877
- Rasheed F, Saeed M, Alikhan NF, Baker D, Khurshid M, Ainsworth EV, et al. Emergence of resistance to fluoroquinolones and third-generation cephalosporins in Salmonella typhi in Lahore, Pakistan. Microorganisms. 2020;8(9):1-9. doi: 10.3390/microorganisms8091336
- Hamidian M, Tajbakhsh M, Walther-Rasmussen J, Zali MR. Emergence of extended-spectrum beta-lactamases in clinical isolates of Salmonella enterica in Tehran, Iran. Jpn J Infect Dis. 2009;62 5:368-371.
- Yoon HJ, Cho SH, Kim SH. A case of multidrug-resistant Salmonella enterica serovar Typhi treated with a bench to bedside approach. Yonsei Med J. 2009;50(1):147-151. doi: 10.3349/ymj.2009.50.1.147
- Ghurnee O, Ghosh AK, Abony M, Aurin SA, Fatema AN, Banik A, et al. Isolation of multi-drug resistant (MDR) and extensively drug resistant (XDR) Salmonella typhi from blood samples of patients attending tertiary medical centre in Dhaka city, Bangladesh. Adv Microbiol. 2021;11(9):488-498. doi: 10.4236/aim.2021.119036
- Qamar A, Ismail T, Akhtar S. Prevalence and antibiotic resistance of Salmonella in South Punjab-Pakistan. PLoS One. 2020;15(11):1-15. doi: 10.1371/journal.pone.0275948
- Krishna D, Dhanashree B. Antibiogram, virulence genes, and biofilm-forming ability of clinical Salmonella enterica Serovars: an in vitro Microb Drug Resist. 2021;27(7):871-878. doi: 10.1089/mdr.2020.0419
- Qamar FN, Yousafzai MT, Dehraj IF, Shakoor S, Irfan S, Hotwani A, et al. Antimicrobial resistance in typhoidal salmonella: surveillance for enteric fever in Asia project, 2016–2019. Clin Infect Dis. 2020;71(Supplement_3):S276-S284. doi: 10.1093/cid/ciaa1323
- Akram J, Khan AS, Khan HA, Gilani SA, Akram SJ, Ahmad FJ, et al. Extensively drug-resistant (XDR) typhoid: evolution, prevention, and its management. Biomed Res Int. 2020;2020:1-7. doi: 10.1155/2020/6432580
- Chatham-Stephens K, Medalla F, Hughes M, Appiah GD, Aubert RD, Caidi H, Angelo KM, Walker AT, Hatley N, Masani S, Nash J. Emergence of extensively drug-resistant Salmonella typhi infections among travelers to or from Pakistan—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2019; 68(1):11-13. doi: 10.15585/mmwr.mm6801a3
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
