By Mansoor Ahmer Khan, Syed Ali Haider, Shahab Mehmood, Aasma Moin Khan
Faculty of Life Sciences, Shaheed Zulfikar Ali Bhutto Institute of Science and Technology (SZABIST), Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD12-1/009
ORCID iD: 0000-0002-1481-6057
How to cite: Khan MA, Haider SA, Mehmood S, Khan AM. Curcumin has Curative Effect on Isoniazid-Rifampicin Induced Hepatotoxicity. Pak J Med Dent. 2023;12(1): 46-52. doi: 10.36283/PJMD12-1/009
Background: Anti-tuberculosis drugs-induced hepatotoxicity is associated with oxidative stress. Curcumin is a powerful antioxidant and has been found to protect the liver from the damaging effects of oxidative stress. The study aimed to assess the curative and protective effects of Curcumin against hepatotoxicity induced by anti TB- drugs (Isoniazid-Rifampicin) by using an experimental model of Albino rabbits.
Methods: Albino rabbits(n=24) were divided into four groups. Group A was the control group, Group B administered Isoniazid (INH) (50 mg/kg/d) and Rifampicin (RMP) (100 mg/kg) alone, Group C received both INH+RMP and Curcumin (60 mg/kg) before and during induction. Group D received INH (50 mg/kg/d) and RMP (100 mg/kg/d) for 7 days, followed by 7 days of Curcumin (60 mg/kg/d). Biochemical testing and liver morphological histopathology was done for all groups. All values were recorded in mean ± standard deviation.
Results: Anti-Tuberculosis drugs increased Alanine Transaminase (ALT), Aspartate Transaminase level (AST), Alkaline Phosphatase level (ALP), Total Bilirubin, and Albumin 62.0±2.5, 172.5±1.0, 128±1.5, 0.80±0.05, 5.00±0.5 respectively and decreased Total protein levels (2.05±1.0). Whereas, Curcumin lowered liver enzymes 37.0±2.8, 126.12±1.5, 90.5±1.0, 0.40±0.01, 3.50±0.5 respectively, and increased levels of total protein (5.00±0.5). Group A exhibited normal liver morphology, whereas, Group B had ballooning degeneration, focal cell necrosis, and liver inflammation. Group C had moderate fatty liver but no centrilobular degeneration or focal cell necrosis and Group D exhibited minor liver inflammation and normal liver morphology.
Conclusion: Curcumin was found preventative and therapeutic remedy which can be used for the treatment of hepatotoxicity.
Keywords: Curcumin; Hepatotoxicity; Hepatoprotective; Isoniazid; Rifampicin.
Tuberculosis (TB) is an infectious and communicable illness caused by Mycobacterium that continues to endanger worldwide public health1. In 2019, around 10 million individuals were diagnosed with TB, and 1.4 million died as a result of the disease2. On the other hand, Isoniazid’s introduction in the 1950s offered an excellent method of TB control. It is still the first-line treatment for both active TB and tuberculosis prevention. Despite its great antibacterial activity, its usage is limited due to significant hepatotoxicity and fatal liver damage. As a result, it is critical to identify the hepatotoxicity mechanism as well as novel treatment targets and more effective techniques to prevent isoniazid-induced hepatotoxicity3,4.
One of the most common drugs used in TB treatment includes Isoniazid (INH) and Rifampicin (RMP) which are used in combination to have an effective TB treatment but increase the risk of hepatic injury5. Hepatotoxicity which is caused by TB medications can influence the hepatocytes or vasculature and affect the biliary epithelium, but the fundamental component and contributing elements which causes hepatic harm are not known. Brief and asymptomatic expanded transaminase levels can be minor and have non-dynamic harm to mitochondria, cell layers, or different structures of hepatocytes6. Furthermore, different studies have been carried out to develop effective ways to reduce the hepatotoxicity of anti-TB drugs, which can be done by either using synthetic drugs or natural botanical products without interfering with their therapeutic actions7.
Curcumin has been used which is a natural product. Contrarily, curcumin, a yellow substance generated and derived from the Curcuma longa plant, is a very potent antioxidant and has potent anti-inflammatory properties8,9. According to a previous study, it was found that Curcumin had a protective effect against chronic liver failure as it helps to reduce harmful oxidative stress10. Due to Curcumin’s chemical structure, it is a strong antioxidant agent that can prevent free radicals11. The expected preventive or putative effects of Curcumin have also been associated with its antioxidant property12. Therefore, this study aimed to assess the curative and protective effects of Curcumin against hepatotoxicity induced by anti TB- drugs in Albino rabbits.
This experimental study was conducted at Shaheed Zulfiqar Ali Bhutto Institute of Science and Technology (SZABIST) in the faculty of Life Sciences. Initially, rabbits (n=24) were kept in polypropylene cages with stainless steel tops, in the SZABIST animal room to protect them from intense sunlight, and the cage bases were lined with paddy husk. It was ensured that the environmental conditions must be controlled. The temperature was maintained between 25-30℃, and the relative humidity at 55%-75%. While, the identification of the rabbits was done by marking their cages with their specific groups, and the rabbits corresponding to each group had been marked and the groups have been assigned randomly.
Albino rabbits of both genders were taken, which weighed an average of 1.2 kg. Rabbits were kept for acclimatization for 10 days under controlled temperature and light cycle. Normal feed and water were given for the entire period. After the acclimatization period, the rabbits were separated into 4 groups, with 6 rabbits in each group; Group A in which rabbits were given normal feed, and in Group B the rabbits were induced to hepatotoxicity by a combination of two drugs; isoniazid and rifampicin orally as per their body weight for 7 days. Group C rabbits received Curcumin for 3 days as a pretreatment, and after 3 days hepatotoxicity was induced by drug combination and Curcumin was also given throughout the induction period simultaneously to check the protective effects. Group D received rifampicin and isoniazid for initial 7 days and was then given Curcumin for 7 days to check the curative effects of Curcumin against hepatotoxicity.
Isoniazid and Rifampicin, the two medications, are administered in combination to have significantly stronger effects in the effort to treat tuberculosis. Isoniazid was set to be administered in doses of 50 mg per kilogram of rabbit weight, whereas rifampicin was set to be given in doses of 100 mg per kilogram of rabbit weight. However, 60 mg of curcumin per kilogram of the rabbits’ weight was given. A combination of INH and RMP was used to cause liver damage, these drugs were given orally for 7 days to induce hepatotoxicity. After 7 days, blood samples were collected to check whether hepatotoxicity was induced or not. A Liver Functioning Test was done to see whether the level of liver enzymes and proteins was elevated or decreased. The levels were compared with the control group.
After completion of the model, blood (2 ml) was drawn from rabbits’ lateral ear veins and the samples were collected in a yellow top vacutainer and labeled according to the groups. Xylene was applied on the rabbits’ ear for visibility of the vein. To obtain serum, blood was centrifuged for 15 minutes at 2200- 2500 RPM. The liver function test was assessed by levels of Albumin, Alanine Transaminase level (ALT), Aspartate Transaminase level (AST), Alkaline Phosphatase level (ALP), total bilirubin and total protein. The levels were compared with the control group to check the intensity of liver damage. The liver of rabbits was isolated and preserved in 10% formalin. Sections of 5 μM were prepared; Hematoxylin and eosin were used to stain slides and then they were observed under a light microscope. Statistical Package for Social Science program (SPSS) by IBM was used to perform statistical analysis. All tests were compared with Group A and all data was expressed as mean and standard deviation (±).
Out of the all groups of rabbits (n=24), experimental group C had decreased average liver weights compared to group D. Figure 1 represents the average liver weight of each group. However, the protective group had a mean liver weight that was more comparable to the control group. The raised hepatic weight of group D is a clear physical sign of hepatotoxicity.
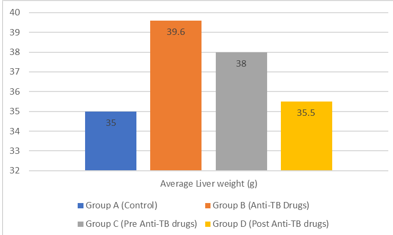
Figure 1: Mean hepatic weight of each group.
During the pharmacological induction of hepatotoxicity, an observational characterization of the feces and urine of all groups was carried out. Following the induction of hepatotoxicity in Group D, another observation analysis was carried out within the allotted time frame of fourteen days, At the end of the 14th day of the curcumin treatment, the feces were more Cecotropes and sticky when compared to the feces that were produced by Group A. After seven days Group B (Anti-Tb Drug) experienced watery diarrhea and red, cloudy urine which can be an indication of hematuria. Group C, on the other hand, was characterized by its small size, mixed shape, and dry, and dark-colored feces (Table 1). While it was discovered that the color of urine was amber and possessed turbidity, whereas group A, includes urine that is transparent with a pale white color, and normal stool consistency. This demonstrates that there had been internal modifications in the gastrointestinal tract and urinary tract of rabbits in which hepatotoxicity was induced.
Table 1: Observational analysis of faces and urine.
| Group A (Control) | Stool Consistency | Urine Coloration |
| Day 1 | Round consistent shape and amount | Pale white, clear |
| Day 3 | Round consistent shape and amount | Pale white, clear |
| Day 5 | Round consistent shape and amount | Pale white, clear |
| Day 7 | round consistent shape and amount | Pale white, clear |
| Group B (Anti-Tb Drug) | Stool Consistency | Urine Coloration |
| Day 1 | Round consistent shape and amount | Pale white, clear |
| Day 3 | Cecotropes, sticky | Pale white, turbid |
| Day 5 | Sticky/slimy | Amber, turbid |
| Day 7 | watery diarrhea | Red, turbid |
| Group C (Pre-Anti TB Drugs) | Stool Consistency | Urine Coloration |
| Day 1 | Round consistent shape and amount | Pale white, clear |
| Day 3 | Cecotropes, slimy | Pale white, clear |
| Day 5 | Cecotropes, slimy | Amber, turbid |
| Day 7 | Small, mixed shape, dry and dark | Amber, turbid |
| Group D (Post-Anti-TB Drugs) | Stool Consistency | Urine Coloration |
| Day 1 | Round consistent shape and amount | Pale white, clear |
| Day 3 | Cecotropes, sticky | Pale white, turbid |
| Day 5 | Sticky/slimy | Amber, turbid |
| Day 7 | watery diarrhea | Red, turbid |
| Day 9 | Cecotropes, slimy | Pink, turbid |
| Day 11 | Cecotropes, sticky | Amber, turbid |
| Day 14 | Cecotropes, sticky | Amber, clear |
Group B expressed that INH and RMP elevated the levels of ALP, AST, ALT, total bilirubin, Albumin (62.0±2.5,172.5±1.0,128±1.5,0.80±0.05,5.00±0.5 respectively) and decreased total protein levels (2.05±1.0) as compared to Group A. Whereas Group C receiving co-administration of Curcumin and INH-RMP and Group D receiving Curcumin after hepatotoxicity induction showed decreased levels of liver enzyme (37.0±2.8,126.12±1.5,90.5±1.0,0.40±0.01,3.50±0.5 respectively) increased levels of total protein (5.00±0.5) when compared to Group B as shown in Table 2.
Table 2: Effects of curcumin on enzyme and protein levels of the liver in isoniazid-rifampicin induced hepatic toxicity in rabbits.
| Groups | Alanine transaminase (ALT)
(IU/L) Mean±SD |
Aspartate transaminase AST
(IU/L) Mean±SD |
Alkaline phosphatase (ALP)
(IU/L) Mean±SD |
Total
Bilirubin (mg/dl) Mean±SD |
Total Protein
(g/dl) Mean±SD |
Albumin
(g/dl) Mean±SD |
| Group A (control) | 22.5±2.0 | 114±0.5 | 48.5±2.0 | 0.23±0.10 | 7.30±1.0 | 3.00±0.2 |
| Group B (Anti-TB drugs) | 62.0±2.5 | 172.5±1.0 | 128±1.5 | 0.80±0.05 | 2.05±1.0 | 5.00±0.5 |
| Group C (Pre-Anti TB drugs) | 49.0±2.4 | 132±1.0 | 79.5±0.5 | 0.50±0.05 | 5.10±1.0 | 3.50±0.1 |
| Group D (Post-Anti TB drugs) | 37.0±2.8 | 126.12±1.5 | 90.5±1.0 | 0.40±0.01 | 5.00±0.5 | 3.50±0.5 |
In addition, Figure 2 represented histological images in which Group A showed normal liver morphology whereas Group B in which hepatotoxicity was induced showed ballooning degeneration, focal cell necrosis and liver inflammation, Group C was pre and co-treated with Curcumin indicated no centrilobular degeneration or focal cell necrosis however it only had mild fatty liver. Group D was post-treated with Curcumin which indicated mild liver inflammation and normal liver morphology
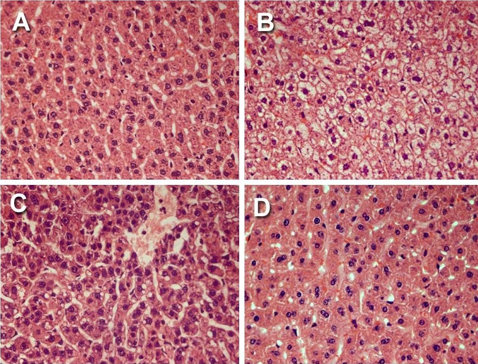
Figure 2: Photomicrograph of a control group (A); Photomicrograph of induction (INH+RMP) group (B); Photomicrograph of pre and co-administration of Curcumin + INH and RMP group (C); Photomicrograph of a post-Curcumin group (D).
The present study found that Curcumin lowered liver enzymes and increased total protein in rabbits, whereas an anti-tuberculosis drug increases liver enzymes. However, tuberculosis is an infectious disease that can last for months or years and is caused by the mycobacterium tuberculosis bacteria. It has been linked to a high death rate throughout history and continues to do so today12. In the beginning, its pathological and anatomical presence was found by Francis Sylvius in 1679. He documented tubercles, abscesses development, and how they led to cavitations and emphysema of the lung. In the year 1699, the republic of Lucca proclaimed the illness to be communicable13. In the year 1735, the health board issued an order mandating the isolation of consumptives before their admittance into public health facilities. These centers were constructed locations to provide treatment14. In the 18th century, tuberculosis (TB) spread over Western Europe and caused an epidemic that resulted in 900 fatalities for every 100,000 people. This earned TB the nickname “the robber of youth” 15.
Isoniazid and rifampicin, which are the first-line treatments for tuberculosis, cause few significant changes in the serum transaminase levels but cause hepatotoxicity16. In this study it was also found, Group B expressed high levels of liver enzymes (ALP, AST, ALT) and Total bilirubin, Albumin which was like the research conducted on animals which suggested that when drugs used to treat tuberculosis are taken in hazardous doses, there is an increase in ALT, AST, and ALP in the blood, which affects the integrity of the hepatocellular phospholipid bilayer and its organelles17. This was also supported by other studies when the animals were given the drugs18,19. According to the findings of the research, the most common cause of liver injury generated by the medication was the administration of a high dosage of acetaminophen, which also significantly elevated the levels of ALT, ALP, and AST in the serum. The blood levels of ALT, ALP, and AST were all significantly lowered by Hedera helix leaf extract, which provided hepatoprotective benefits as well as antioxidant characteristics against acetaminophen-induced liver damage20.
Hepatitis caused by isoniazid and rifampicin is linked to the death of cells in certain regions of the liver as well as centrilobular degeneration21. The study found isoniazid and rifampicin had been observed to produce hepatotoxicity in rabbit liver cells, and microscopic examination of these cells indicates inflammation and cell death at focal and centrilobular regions22,23. This was following the characteristics observed in this study which validate the current study animal model. The buildup of a toxic intermediate of isoniazid and rifampicin metabolism is thought to be the cause of liver damage24. Thrombocytopenia can be caused by any of the basic anti-tubercular medicines25. It happens as a hematological response in the case of isoniazid. One of the indications of thrombocytopenia is blood in the rabbit’s urine or stool26. Although thrombocytopenia is uncommon, it can occur with anti-tubercular medications such as Rifampicin and Isoniazid, which can have deadly side effects. Bilirubin is not effectively processed due to hepatotoxicity or a damaged liver27. Thus, drastically elevated bilirubin levels in the blood can induce jaundice. A high level of bilirubin in the circulation can induce leakage and spread into adjacent tissues28-30. In line with this, another research demonstrated comparable biochemical changes, such as elevated serum ALT levels, in rabbits who were subjected to INH-induced hepatotoxicity, indicating aberrant functioning integrity of the cells that make up the liver29.
In this study, Curcumin after hepatotoxicity induction showed decreased levels of liver enzyme, however, curcumin and its derivatives have been shown in studies to have antimycobacterial and antitubercular activity via influencing the human immune response throughout infection. While recent trials on curcuminoids have shown that they are safe for human intake, an examination of the derivatives is required. The goal of this study was to assess the role in the treatment of curcumin and its compounds in tuberculosis. As a result, the current study provides evidence that curcumin might be regarded as a potent antitubercular agent and may be exploited to develop innovative medications for TB protection and therapy. While rising evidence supports the use of curcumin and its derivatives for tuberculosis therapy, further clinical and preclinical research is needed before recommending curcumin formulations in public health30.
This study found curcumin as a protective and curative medication for preventing liver failure by tuberculosis medications-induced hepatotoxicity. Therefore, curcumin is a useful anti-oxidant in the treatment of hepatotoxicity.
The authors would like to acknowledge Prof. Dr. Kashif Ali, Dean of Faculty Life Sciences SZABIST, for his mentorship and direction during the project, as well as Mr. Mazhar Ayaz for his assistance with animal care.
There was no conflict of interest.
The Ethics Reviewing Board at Shaheed Zulfiqar Ali Bhutto Institute of Science and Technology approved this study, according to the declaration of Helsinki principles.
The research study was funded by the Shaheed Zulfikar Ali Bhutto Institute of Science and Technology (SZABIST).
MAK contributed to the conception and design of the study, the input of intellectual data and the drafting of the manuscript. SAH assisted in the acquisition, analysis and interpretation of data and paper drafting. SM was the supervisor of the study while AMK helped in the sample analyses for biochemistry and histopathology results. All authors approved the final version of the manuscript to be published.
- Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. Dtsch Arztebl Int. 2019;116(43): 729-735.
- Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8(1):1. doi: 10.1016/S2213-2600(19)30418-7
- Li Y, Luo WW, Cheng X, Xiang HR, He B, Zhang QZ, et al. Curcumin attenuates isoniazid‐induced hepatotoxicity by upregulating the SIRT1/PGC‐1α/NRF1 pathway. J Appl Toxicol. 2022:1192-1204. doi: 10.1002/jat.4288
- Taiwo J, Shemishere U, Omoregie E. Assessment of hepatoprotective effect of extracts of Alstonia boonei leaf in isoniazid and rifampicin co-treated rats. World J Biomed PharmSci. 2018;3:1-6. doi: 10.5281/zenodo.3243894
- Tariq S, Khan TS, Malik S, Anwar MS, Rashid A. Frequency of anti-tuberculous therapy-induced hepatotoxicity in patients and their outcome. J Ayub Med Coll Abbottabad. 2009;21(4):50-52.
- Kamil N, Imran-ul-Haque HS. Hepatoprotective Effect of Calotropis procera in Isoniazid and Rifampicin Induced Hepatotoxicity. Pharmacogn J. 2014;6(5):9-14. doi: 10.5530/pj.2014.5.3
- Eminzade S, Uras F, Izzettin FV. Silymarin protects liver against toxic effects of anti-tuberculosis drugs in experimental animals. Nutr Metab. 2008;5(1):1-8. doi: 10.1186/1743-7075-5-18
- Ohashi H, Tsuji M, Oguchi T, Momma Y, Nohara T, Ito N, et al. Combined treatment with curcumin and ferulic acid suppressed the Aβ-induced neurotoxicity more than curcumin and ferulic acid alone. Int J Mol Sci. 2022;23(17):1-20. doi: 10.3390/ijms23179685
- Rukoyatkina N, Shpakova V, Bogoutdinova A, Kharazova A, Mindukshev I, Gambaryan S. Curcumin by activation of adenosine A2A receptor stimulates protein kinase a and potentiates inhibitory effect of cangrelor on platelets. Biochem Biophys Res Commun. 2022; 586:20-26. doi: 10.1016/j.bbrc.2021.11.006
- Tu CT, Yao QY, Xu BL, Wang JY, Zhou CH, Zhang SC. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: modulation of high-mobility group box 1, Toll-like receptor 4 and 2 expression. Food Chem Toxicol. 2012;50(9):3343-3351. doi: 10.1016/j.fct.2012.05.050
- Prasad S, DuBourdieu D, Srivastava A, Kumar P, Lall R. Metal–curcumin complexes in therapeutics: an approach to enhance pharmacological effects of curcumin. Int J Mol Sci. 2021;22(13):1-24. doi: 10.3390/ijms22137094
- Derkho MA, Sereda TI, Mukhamedyarova LG. Environmental and epidemiological features of tuberculosis. In Ecological Agriculture and Sustainable Development 2019 (pp. 203-210). Available from: https://www.elibrary.ru/item.asp?id=39141659
- de Farias Gabriel A, Kirschnick LB, Só BB, Schuch LF, Silveira FM, Martins MA, et al. Oral and maxillofacial tuberculosis: A systematic review. Oral Dis. 2022:1-10. doi: 10.1111/odi.14290
- Panezai AJ, Khan M, Ishaque SM, Ullah R, Khan AA, Rahat N. Variable doses of Nigella sativa in isoniazid induced liver toxicity in rabbits. Pak-Euro J Med Life Sci. 2022;5(2):187-194. doi: 10.31580/pjmls.v5i2.2405
- Rehman U, Syed QA, Asghar HA, Arshad MK, Sultan G, Asghar A, Aslam M, Abdullah M. Alimentary and Recuperative Prospective of Curcuma longa (Turmeric). Sch Int J Biochem. 2022;5(5):67-75.
- Ronald LA, FitzGerald JM, Bartlett-Esquilant G, Schwartzman K, Benedetti A, Boivin JF, et al. Treatment with isoniazid or rifampin for latent tuberculosis infection: population-based study of hepatotoxicity, completion and costs. Eur Respir J. 2020;55(3):1-12. doi: 10.1183/13993003.02048-2019
- Sabina EP, Peter S J, Geetha A. A comparison of hepatoprotective activity of Bacoside to Silymarin treatment against a combined Isoniazid and Rifampin-induced hepatotoxicity in female Wistar rats. J Histotechnol. 2019;42(3):128-136. doi: 10.1080/01478885.2019.1638535
- Barberis I, Bragazzi NL, Galluzzo L, Martini M. The history of tuberculosis: from the first historical records to the isolation of Koch’s bacillus. J Prev Med Hyg. 2017; 58(1): E9-E12.
- Xu N, Yang JX, Yang J. Incidence and associated risk factors of antituberculosis drug-induced hepatotoxicity among hospitalised patients in Wuhan, China. Eur J Hosp Pharm. 2022;29(4):217-221. doi: 10.1136/ejhpharm-2020-002433
- Li G, Yang Y, Yang J, Suo Y, Xu H, Liu P, et al. Hepatoprotective effects of Malus hupehensis tea against isoniazid-and rifampicin-induced liver injury by regulating cytochrome P450 in mice. J Funct Foods. 2021;84:1-7. doi: 10.1016/j.jff.2021.104580
- Zodape GV, Bhise PP. Effect of aloe vera extract and isoniazid-rifampicin drug on liver histological studies of male Wistar rats. Int J Pharm Sci Res. 2018;9(10):4318-4325. doi: 10.13040/IJPSR.0975-8232.9(10).4318-25
- Ali S, Ali U, Bangulzai MA, Malik H, Anwer A, Abbas M. Hepatoprotective effects of ivy leaves extract in isoniazid induced hepatotoxicity in rabbits. Rawal Med J. 2022;47(2):494-497.
- Sarich TC, Zhou T, Adams SP, Bain AI, Wall RA, Wright JM. A model of isoniazid-induced hepatotoxicity in rabbits. J Pharmacol Toxicol Methods. 1995;34(2):109-16. doi: 10.1016/1056-8719(95)00044-I
- Li F, Zhou J, Li Y, Sun K, Chen J. Mitochondrial damage and Drp1 overexpression in rifampicin-and isoniazid-induced liver injury cell model. J Clin Transl Hepatol. 2019; 7(1): 40-45. doi: 10.14218/JCTH.2018.00052
- Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26. COV2. S vaccination. N Engl J Med. 2021;384(20):1964-1965. doi: 10.1056/NEJMc2105869
- Maurício J, Flor-de-Lima B, Pacheco P. Severe rifampicin-induced thrombocytopenia in a patient with miliary tuberculosis. Pulmonology. 2019:1-3. 2019. doi 10.1016/j.pulmoe.2019.09.005
- Naji KM, Al-Khatib BY, Al-Haj NS, D’souza MR. Hepatoprotective activity of melittin on isoniazid and rifampicin induced liver damage in male albino rats. bioRxiv. 2020:1-31. doi: 10.1186/s40360-021-00507-9
- Phillips P. Mechanisms of Rifampicin-induced Hepatotoxicity in Pregnane X Receptor Humanized Mice (Doctoral dissertation preview, North Carolina Central University,). 2020: 1-10. Available from: https://www.proquest.com/openview/44b230eec78da466b3f9e4f2b6bb0e6e/1?cbl=18750&diss=y&pq-origsite=gscholar&parentSessionId=iM%2B6kq94L75ytoPyJm4s3tYj5Tj6M%2BXOL%2FjGWDjH0Ro%3D
- Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70(6):2204-2215. doi: 10.1002/hep.30824
- Wu S, Wang M, Zhang M, He JQ. Metabolomics and microbiomes for discovering biomarkers of antituberculosis drugs-induced hepatotoxicity. Arch Biochem Biophys. 2022;716:109118. doi: 10.1016/j.abb.2022.109118
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
