By Urooj Gul Samoo1, Shaista Ehsan2
AFFLIATIONS:
- National Institute of Cardiovascular Diseases (NICVD), Karachi, Pakistan.
- Department of Pediatrics, Ziauddin Medical University, Karachi, Pakistan.
DOI: https://doi.org/10.36283/PJMD11-4/007
ORCID iD: 0000-0003-1074-2035
How to cite: Ehsan S, Samoo UG. Self-Assembled Bubble Continuous Positive Airway Pressure (CPAP) Device as Primary Respiratory Support for Neonatal Respiratory Distress in Low-Resource Settings. Pak J Med Dent. 2022;11(4): 41-49. doi: 10.36283/PJMD11-4/007
Background: Self-assembled, bubble continuous positive airway pressure is a low-cost noninvasive respiratory support modality used to manage newborns with respiratory distress. Very few local studies have been conducted regarding its use in the management of neonatal respiratory distress. This study was conducted to evaluate the effectiveness of this device as a primary treatment modality for neonatal respiratory distress.
Methods: This cross-sectional study was conducted in the Department of Pediatrics, Ziauddin Hospital, Karachi from 1st February 2018-31st July 2018 on neonates (n=200) admitted with respiratory distress, in whom self-assembled bubble continuous positive airway pressure was used as a primary respiratory support. The effectiveness of its use was determined, based on the absence of associated complications, signs of respiratory distress, and oxygen saturation at room air of 94% or more. Data were analyzed by SPSS version 20. The Chi-square test was used for categorical variables and a p-value <0.05 was considered statistically significant.
Results: The mean gestational age was 34.3+2.73 weeks, the mean age at initiation of bubble continuous positive airway pressure was 7.7 4 hours, and the mean duration of hospital stay was 4.2 days. The observed failure rate in the study population was 8.5%, while the mortality was only 2%. The finding of respiratory distress syndrome on chest- X-ray with (FiO2) > 60% was observed. In addition, bubble CPAP after 6 hours of the onset of respiratory distress showed significant results (p<0.001).
Conclusion: Self-assembled bubble continuous positive airway pressure is a significant and effective treatment modality (p=0.001) in neonatal respiratory distress.
Keywords: Bubble Continuous Positive Airway Pressure; Neonates; Respiratory Distress.
Respiratory distress is a major cause of morbidity and mortality in neonates and frequently requires invasive ventilation. Neonates suffering from this condition are commonly managed through oxygen inhalation, surfactant therapy, Continuous Positive Airway Pressure (CPAP), and mechanical aeration depending on the extent and severity of the illness and the neonates’ response to treatment1,2. A wide range of CPAP devices are commercially available and are extensively used for facilitating gaseous exchange and lung function3.
The presence of respiratory distress in neonates is life-threatening and the major risk factors for this condition e.g., sepsis; low birth weight and prematurity are more prevalent in low- and middle-income countries (LMIC)4,5. Even though a significant reduction in mortality in the under 5 years age group has been observed in the low- and middle-income countries in the last two decades, neonatal mortality remains high. In resource-limited settings, a cost-effective intervention such as self-assembled Bubble CPAP (B-CPAP) may be preferable to invasive ventilation based on its cost-effectiveness and lesser requirement for training6,7.
The use of self-assembled bubble CPAP helps to splint the upper airway by providing positive airway pressure while decreasing apnea and obstruction that in turn facilitates the lungs to expand and prevent alveolar collapse8. Underwater bubble CPAP and ventilator-driven CPAP have been identified as two of the widely used CPAP models with the difference in pressure sources. Bubble CPAP has been known to offer superior results in comparison to the conventional CPAP models in terms of the fact that the expiratory limb is submerged in an underwater seal, which is responsible for the oscillatory vibrations in the chest as the flow of gas, is transmitted by the infant’s airway. The vibrations simulate the waveforms that are caused by high-frequency vibrations9.
Bubble CPAP is regarded as a non-invasive technique that is frequently used in low-resource settings for treating RDS10,11. The advantages of using bubble CPAP include a lesser risk of lung injury and trauma as compared to mechanical ventilation injury and consequently a shorter duration of hospitalization12. Furthermore, in low- and middle-income countries it is a more feasible modality because of its cost-effectiveness and ease of usage. Though research studies have recommended the use of bubble CPAP in all neonates with respiratory distress regardless of the gestational age some research literature has reported that preterm infants with less than 28 weeks of gestation are less likely to gain benefit from ventilation following bubble CPAP as the primary respiratory support as compared to surfactant administration and brief invasive ventilation followed by CPAP13,14.
Only a few research studies have been conducted to assess the outcome of the locally assembled bubble CPAP. There is a need to collect more data on the effectiveness of its use in resource-limited settings to treat neonates with respiratory distress and to determine the most appropriate gestational age group that responds effectively to its use13. This study was, therefore, conducted to assess the outcome of neonates admitted with respiratory distress by making use of a locally assembled bubble CPAP which holds promise to minimize the need of the mechanical ventilation as well as invasive surfactant therapy, thereby, serving as a cost-effective modality. Moreover, there is also a dearth of research data on different variables, which serve as predictors of success or failure of the modality within a limited resource setup15. The objective of this study was to evaluate the effectiveness of self-assembled bubble CPAP as a primary treatment modality for neonates with respiratory distress and to determine the frequency of associated complications when this non-invasive treatment modality is applied.
This cross-sectional study was carried out for six months, from 1st February 2018 to 31st July 2018. Approval for the study was sought from the Ethics Review Committee of Ziauddin University hospital (Ref no. 0290917UGPEDS). A total of 200 neonates admitted to the Neonatal Intensive Care Unit (NICU) with respiratory distress and in whom B-CPAP was used as primary respiratory support were included. The sample size was calculated using the WHO calculator by taking a proportion of success of B-CPAP at 84.5% with a margin of error of 5% at a 95% confidence interval. Sampling was done through a non-probability convenience sampling technique.
The inclusion criteria were based on the presence of the clinical findings of increased respiratory rate i.e., >60 breaths/minute plus any of the following features: grunting, subcostal/intercostal retractions, nasal flaring, inability to maintain spO2 >90% at room air and typical findings of respiratory distress syndrome on chest X-ray i.e., ground glass appearance or white out. However, all neonates suffering from congenital anomalies, heart failure, having a Downes score of >7 at admission, those requiring mechanical ventilation at the time of admission, and those who were discharged from the hospital against medical advice were excluded from the study.
The B-CPAP device was made using an oxygen flow meter as the oxygen source. The inspiratory limb of the B-CPAP was attached to the oxygen flow meter and the oxygen flow was adjusted with the flow regulator. With the bubble CPAP device, blended and humidified oxygen was delivered via short nasal prongs and pressure in the circuit was maintained by immersing the distal end of the expiratory tubing in water. These short binasal prongs were applied to the neonate with the help of an adhesive bandage on both cheeks. It was ensured that binasal prongs make an appropriate seal. An orogastric tube was placed for gastric decompression. A 1000 ml bottle was filled with sterile water and marks were made on it at a 1cm distance with ‘10’ marked at the base and ‘0’ marked at the water interface. Continuous positive end-expiratory pressure was started at 5 cm of water. The fraction of inspired oxygen (FiO2) was adjusted to maintain SpO2 >90% and the flow titrated to produce continuous bubbling.
The clinical assessment of the bubble CPAP effectiveness was based on the improvement in Downes score, absence of signs and symptoms of respiratory distress as well as not having additional requirement of oxygen and oxygen saturation at room air of 94%. The criteria for weaning off from B-CPAP included minimal or no chest retractions or absence of respiratory distress (respiratory rate ranging between 30 – 60 breaths /min) and SpO2 > 90% with PEEP < 5cm of water. Whereas, failure of the B-CPAP was considered in case of persistence of hypoxia in the neonates i.e., SpO2 < 87% with PEEP > 7cm of the water level, presence of sub-costal as well as the intercostal recessions with PEEP > 7cm of the water level, prolonged apnea (≥ 20 sec) or recurrent episodes of apnea with more than 2 apnea episodes in less than 24 hours that required ambo bagging, a Downes score of 7 or > 7, and any specific condition of shock that required inotrope support16. The success of the treatment modality was consistently examined to monitor for any adverse reactions or problems. Those study participants who failed to respond to bubble CPAP were intubated and mechanical ventilation started.
Information regarding neonatal variables was recorded on a structured pre-designed proforma. These included demographic data such as birth weight, gender, gestational age, underlying etiology of respiratory distress, Downes score at start, after 6 hours of starting bubble- CPAP and after 12 hours of its use, duration of hospital stays, maternal age, mode of delivery, twin gestation, regular antenatal visits, administration of antenatal steroids, maternal risk factors such as hypertension, diabetes and prolonged rupture of membranes. Patient data were also stratified into four groups as age <30 weeks gestation, 31-35 weeks, 36-40 weeks, and gestational age 41weeks and above. Furthermore, the age at which bubble CPAP was initiated, duration of bubble CPAP usage, length of hospital stay, outcome and complications of bubble CPAP usage were also recorded.
Informed written consent was obtained from the parents and the confidentiality of patients was maintained by a coding system. Data was analyzed using SPSS version 20. Qualitative data was expressed as mean and standard deviation and quantitative data as frequencies and percentages. Chi-square was applied for categorical variables. To adjust for many outcome variables, responses between groups were considered statistically significant at a p-value <0.05.
A total of 200 neonates admitted to the neonatal intensive care unit (NICU) with respiratory distress, were included in the study and a self-assembled bubble CPAP device (B-CPAP) was used as primary respiratory support for them. The mean birth weight of the study population was observed to be 2.14 ± 0.46 kilograms with a range of 1 to 4.6 kilograms. The mean gestational age of the neonates was observed to be 34.3 ± 2.73 weeks. The mean maternal age was 29.48 ± 2.18 years with a range of 20 to 37 years. Furthermore, the mean age at which the bubble CPAP was initiated was 7.7 ± 4 hours and the mean total duration of bubble CPAP usage was 57.9 ± 35.5 hours. The mean length of hospital stay was noted to be 4.2 days with a range of 1 to 30 days. The study population comprised 116 males and 84 females, with an overall male to female ratio of 1.38:1. In most of the neonates i.e., 105 (52.5%), respiratory distress syndrome was the underlying cause of the symptoms, followed by sepsis in 29 (14.5%). Figure 1 shows the frequency of various etiologies of neonatal respiratory distress in the study population.
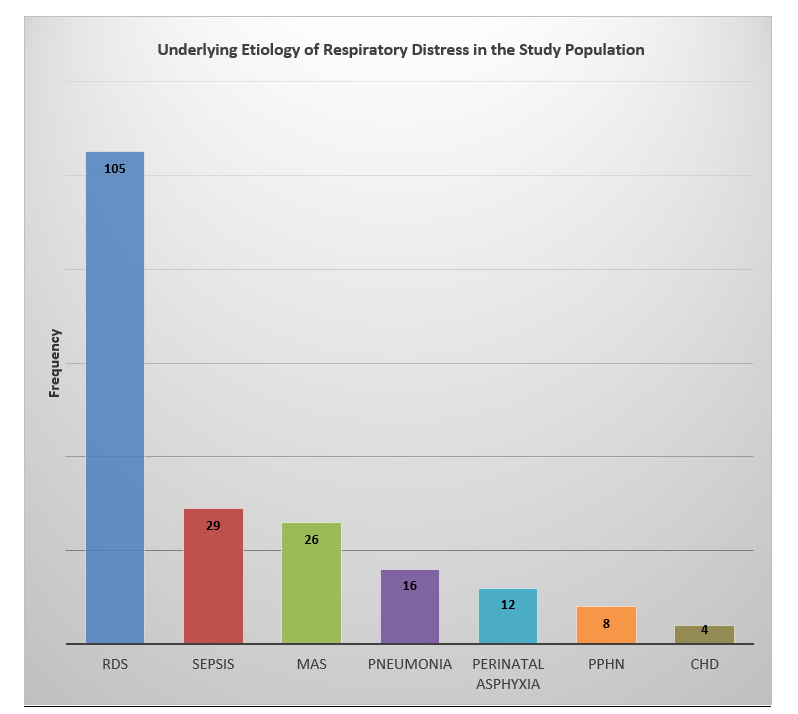
Figure 1: Frequency of various underlying causes of neonatal respiratory distress.
RDS=respiratory distress syndrome, MAS=meconium aspiration syndrome, PPHN=persistent pulmonary hypertension, CHD=congenital heart disease.
The observed failure rate of bubble CPAP in the study population was 8.5%, as 17 neonates in the study population failed to respond to it. Risk factors that were found to be significantly associated with bubble CPAP failure included a mean respiratory rate of > 60/minute, presence of subcostal recessions, cyanosis, a need for resuscitation, typical findings of respiratory distress syndrome on chest- X-ray, a fraction of inspired oxygen (FiO2) > 60% and initiation of bubble CPAP after 6 hours of the onset of respiratory distress (p-value <0.001, 0.004, <0.001,0.001, <0.001, 0.006, >0.001 respectively). Table 1 shows the effect of various neonatal and maternal factors on the outcome of bubble CPAP usage in the study population. With regards to the maternal factors associated with the outcome of bubble-CPAP usage, a significantly positive association was observed between the administration of both doses of antenatal steroids (p-value 0.02) and improvement of respiratory distress after initiation of bubble-CPAP, in the studied neonates. Furthermore, regular antenatal checkups were also significantly associated with the success of bubble-CPAP use in the study population (p-value 0.01). Other maternal variables, such as maternal age, hypertension, diabetes and premature rupture of membranes did not affect the outcome of bubble CPAP in the study population.
Table 1: Neonatal and maternal characteristics with regards to B-CPAP outcome.
| Characteristics | B-CPAP Success (n=183)
n (%) |
B-CPAP Failure (n=17)
n (%) |
p– Value |
| Neonatal Age in Hours at Initiation of
B- CPAP (mean + SD) |
5.8 + 2.50 | 11.5 + 3.5 | 0.61 |
| Duration of Hospital Stay in Days (mean + SD) | 4.8 + 2.1 | 4.1 + 3.8 | 0.09 |
| Male (N= 116) | 57 (49.1%) | 59 (50.9%) | 0.96 |
| Female (N= 84) | 40 (47.6%) | 44 (52.4%) | 0.84 |
| Birth Weight (Kilograms) < 1.5 Kg | 38 (20.8%) | 4 (23.5%) | 0.76 |
| 1.5 Kg – 2 Kg | 64(34.9%) | 6 (35.3%) | 0.81 |
| 2.1 Kg – 3 Kg | 55 (30.1%) | 5 (29.4%) | 0.60 |
| > 3 Kg | 26 (14.2%) | 2 (11.8%) | 0.91 |
| Twin Gestation | 28 (15.3%) | 3 (17.6%) | 0.62 |
| Resuscitated at Birth | 11 (6%) | 7 (41.2%) | 0.001 |
| Mean Respiratory Rate (b/min) | 60.26 + 4 | 66 + 5.4 | < 0.001 |
| Mean heart rate (b/min) | 158.5 + 6.2 | 158.2+ 9 | 0.78 |
| Nasal flaring | 93 (51%) | 13 (76.5%) | 0.062 |
| Subcostal recessions | 128 (69.9%) | 15 (88.2%) | 0.004 |
| Cyanosis | 26 (14.2%) | 6 (35.3%) | <0.001 |
| CXR Suggestive for RDS | 89 (48.6%) | 16 (94.1%) | <0.001 |
| Initiation of B- CPAP < 6hrs | 144 (78.7%) | 3 (17.6%) | <0.001 |
| Initiation of B- CPAP > 6hrs | 39 (21.3%) | 14 (82.4%) | 0.001 |
| FiO2 at 15-20 min of B- CPAP % (mean + SD) | 44.6 + 23 | 68.2 + 25 | 0.006 |
| PEEP at 15-20 min of B- CPAP (mean + SD) | 4.8 + 0.8 | 5.4 + 0.2 | 0.22 |
| Maternal Age (mean in Years) | 29.8 + 4.6 | 28.2 + 3.5 | 0.26 |
| Maternal Hypertension | 5 (2.7%) | 1 (5.9%) | 0.74 |
| Maternal Diabetes | 15 (8.2%) | 2 (11.8%) | 0.76 |
| Regular Antenatal Visits | 164 (89.6%) | 5 (29.4%) | 0.01 |
| Prolonged Rupture of Membranes | 2 (1.1%) | 1 (5.8%) | 0.79 |
| Elective LSCS | 61 (33.3%) | 5 (29.4%) | 0.81 |
| Emergency LSCS | 101 (55.2%) | 9 (52.9%) | 0.90 |
| Antenatal Steroid 1st Dose | 71 (38.8%) | 4 (23.5%) | 0.04 |
| Antenatal Steroid 2nd Dose | 56 (30.6%) | 2 (11.8%) | 0.02 |
| No Antenatal Steroids | 56 (30.6%) | 11 (64.7%) | 0.01 |
CXR= X-ray Chest, B-CPAP= Bubble Continuous Positive Airway Pressure, RDS=Respiratory Distress Syndrome, FiO2=Fractional Inspired Oxygen, PEEP = Positive End Expiratory Pressure, LSCS=Lower Segment Caesarion Section.
Concerning the total number of neonates in each gestational age group, it was noted that the failure rate on bubble-CPAP was the highest in less than the 30-week gestational age group i.e., 3 out of a total 15 (20%) in this age group failed to improve on bubble CPAP. The second highest failure rate was observed in the gestational age group 36-40 weeks wherein, out of a total of 68 neonates, 9 (13.2%) failed to respond to bubble CPAP. Table 2 reveals the success and failure rate of bubble CPAP in relation to the gestational age of the study population.
Table 2: Association of gestational age and bubble CPAP Outcome.
| Parameter | Gestational Age and B-CPAP Outcome | |||
| Gestational Age in weeks | < = 30
15(7.5%) |
31-35
116(58%) |
36-40
68(34%) |
> 40
1(0.5%) |
| Success | 12(80%) | 111(95.7%) | 59(86.8%) | 1(100%) |
| Failure | 3(20%) | 5(4.3%) | 9(13.2%) | 0(0%) |
| p-value | 0.01 | 0.16 | 0.03 | 0.04 |
Continuous Positive Airway Pressure (CPAP).
In 183 out of a total of 200 neonates on bubble CPAP, the Downes score improved with time i.e., decreased in the majority to 4 or <4 after 12 hours to 24 hours of bubble CPAP use while in neonates in whom B-CPAP failed (n=17), the Downes score increased to 6 and >7 after 12 hours and this difference was significant between both groups after 6 and 12 hours (p-value 0.001). Downes score in the first 24 hours of using bubble CPAP in the study population that responded to it, is shown in Table 3.
Table 3: Downes Score in the first 24 hours in neonates with B-CPAP success.
| Downes Score | 1 Hour n (%) | 6 Hours n (%) | 12 Hours n (%) | 24 Hours n (%) |
| 1 | – | – | – | 14 (7.7) |
| 2 | – | – | 18 (9.8) | 82 (44.8) |
| 3 | 2 (1.1) | 18 (9.8) | 68 (37.2) | 51 (27.9) |
| 4 | 8 (4.4) | 92 (50.3) | 64 (35) | 36 (19.6) |
| 5 | 4 (2.2) | 26 (14.2) | 21 (11.5) | – |
| 6 | 82 (44.8) | 43 (23.5) | 12 (6.5) | – |
| 7 | 87 (47.5) | 4 (2.2) | – | – |
| Total | 183 | 183 | 183 | 183 |
Downes Score < 4 = mild respiratory distress; score 4-6 = moderate respiratory distress; score > 6 = severe respiratory distress.
Out of the total study population of 200 neonates on bubble CPAP, 59 (29.5%) neonates had associated complications e.g., sepsis, congenital heart disease, hypoxic ischaemic encephalopathy, pneumonia, and pneumothorax. Associated complications were observed to be significantly high in the gestational age group 36-40 weeks (p-value 0.04). Out of a total of 68 neonates in this age group, 25 (36.8%) developed complications. In addition, a high rate of sepsis was observed in the gestational age group 36-40 weeks, whereas shock as an associated complication was not reported in any of these neonates. Furthermore, in three of the neonates in the gestational age group 36-40 weeks, pneumonia was the underlying cause of respiratory distress. In the gestational age group 31-35 weeks, hypoxic-ischemic encephalopathy as a complication was present in 2 patients. Pneumothorax as a complication of bubble CPAP developed in two neonates one of these was in the gestational age group of 31-35 weeks and the other was between 36-40 weeks of gestation. The association of various complications after initiation of bubble CPAP is shown in Table 4.
With regards to the outcome in the study population, the overall mortality rate was observed to be 2% as 4 neonates out of a total population of 200 expired and all these 4 neonates belonged to the bubble CPAP failure group. Overall, 3 neonates left against medical advice, 2 were referred out and 191 (95.5%) neonates were discharged.
Table 4: Gestational age in weeks and associated complications.
| Complications | Characteristics | Gestational Age in Weeks | n=200 | p-Value | |||
| <30 weeks
Frequency (n) |
31-35 weeks
Frequency (n) |
36-40 weeks
Frequency (n) |
>40 weeks
Frequency (n) |
||||
| Pneumothorax | With Pneumothorax | 0 | 1 | 1 | 0 | 2 | 0.94
|
| Without | 15 | 115 | 67 | 1 | 198 | ||
| Sepsis | With Sepsis | 5 | 11 | 13 | 0 | 29 | 0.72 |
| Without | 10 | 105 | 55 | 1 | 171 | ||
| Shock | With Shock | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Without | 15 | 116 | 68 | 1 | 200 | ||
| Pneumonia | With Pneumonia | 0 | 2 | 3 | 0 | 5 | 0.93 |
| Without | 15 | 114 | 65 | 1 | 195 | ||
| PDA | With PDA | 0 | 0 | 1 | 0 | 1 | 0.96 |
| Without | 15 | 116 | 67 | 1 | 199 | ||
| HIE | With HIE | 0 | 2 | 1 | 0 | 3 | 0.81 |
| Without | 15 | 114 | 67 | 1 | 197 | ||
| Others | Yes | 3 | 6 | 6 | 0 | 15 | 0.76 |
| No | 12 | 110 | 62 | 1 | 185 | ||
This study aimed to highlight the role of bubble CPAP as a treatment modality for neonatal respiratory distress in low-resource settings as most of the evidence regarding the effectiveness and utility of bubble CPAP in neonates is from high-income countries17. Respiratory Distress Syndrome was observed to be the most common cause of respiratory distress in the study population, followed by sepsis and Meconium Aspiration Syndrome. These findings can be explained by the fact that a large majority of the neonates in our study were preterm i.e., 131 out of a total of 200 were of <35 weeks gestational age and therefore were more prone to these conditions. However, in a study on the use of bubble CPAP in neonates by Al-Lawama et al. The most frequent etiology of respiratory distress was transient tachypnea of the newborn (TTN) seen in 42% of the study population18.
In our study, antenatal steroids were used in 66.5% of the neonates and most of the deliveries were booked cases. A study from India by Arora et al. has reported similar results19. This study observed that typical chest X-ray findings suggestive of respiratory distress syndrome (RDS) and a higher fraction of inspired oxygen were associated with failure of bubble CPAP which is like a study on 189 neonates by Kakkilaya et al20. This study observed that a Downes score of 4 or less at the initiation of bubble CPAP is a predictor of success as opposed to a score of 5 or more. These findings are quite like those reported by a study from India study by Urs et al21. Furthermore, the success of our study in managing moderate neonatal respiratory distress can be attributed to the early initiation of B-CPAP at a Downes score of 4.
The observed failure rate of bubble CPAP was the highest (20%) in the gestational age group less than 30 weeks gestation which can be explained by the higher frequency of associated complications in this age group. On the other hand, the neonatal age group of 31–35-week gestation was observed to be ideal for the successful implementation of the bubble CPAP as there were few complications in this group. A high observed failure rate of 13 % on bubble CPAP in the gestational age group 36-40 weeks can be explained by the higher frequency of sepsis in this group. Mustufa et al. have also highlighted sepsis as the leading cause of neonatal morbidity and mortality in developing countries22. The results of our study indicate that the use of bubble CPAP in neonates less than 30-week gestation does not produce significant results. Similarly, several other local studies have reported the use of bubble CPAP to be more effective at a gestational age of more than 30 weeks15,21.
Regarding the adverse events of bubble CPAP, a common concern among clinicians is the occurrence of pneumothorax. However, in this study, only 1% of the study population developed pneumothorax which is like a study by Nahimana et al. wherein, none of the preterm neonates on bubble CPAP developed any complications including pneumothorax23. In our study, the success rate of bubble CPAP was 91.5%, whereas, in contrast, a similar study by Umran et al. observed a success rate of only 44.2%, which is much less than that observed by our study24. In another similar study from Karachi, only 63.6% of the neonates were successfully weaned off from bubble CPAP25. However, like our findings Fatima et al. have reported a success rate of 93.3% after using bubble CPAP in neonates with respiratory distress26. In a study from India by Bahman-Bijari et al., a 100% survival rate at 48 hours for preterm neonates on bubble CPAP was observed, which is comparable to a 98% survival rate in our study27.
Our findings are also comparable to a study by Dewez and van den Broek et al wherein a 75% reduction in respiratory distress-related mortality in low and middle-income countries after the use of bubble CPAP was reported7. The primary outcome of this study was the improvement in the severity of respiratory distress. Furthermore, most of the neonates responded well and did not require resuscitation. Other studies from developing countries have also observed bubble CPAP to significantly reduce the burden of morbidity in neonates28. This study has added to the local research data to prove the effectiveness of bubble CPAP in treating neonates with respiratory distress.
However, the applicability of our study is limited by its being a single-center study as multicenter randomized control trials are required for the confirmation of these study findings. It is recommended that in low-resource settings, the use of bubble CPAP in neonates with respiratory distress be encouraged as it is a promising and cost-effective intervention. However, further research is needed to study the barriers and facilitators of bubble CPAP implementation for practical purposes, especially in low and middle-income countries. Furthermore, future research conducted in the local context must focus on determining the effectiveness of bubble CPAP as primary respiratory support in managing neonates with respiratory distress at the level of primary and secondary healthcare facilities.
In low-resource settings like Karachi, Pakistan, self-assembled low-cost bubble CPAP acts as an effective and treatment modality and has minimal associated complications in neonates with respiratory distress.
The authors would like to thank the Pediatrics Department (Ziauddin University Hospital) for the help in data collection.
The author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Ziauddin University Ethical Review Committee approved the research with the reference number: 0290917UGPEDS.
Verbal and written consents were obtained from the patients.
UG performed the study and conducted the literature search, analysis, and data interpretation. SE conceived the idea, drafted, proofread the manuscript, and completed the final version for publication.
- Anwaar O, Hussain M, Shakeel M, Baig MM. Outcome of use of nasal continuous positive airway pressure through infant flow drivers in neonates with respiratory distress in a tertiary care hospital in Pakistan. J Ayub Med Coll Abbottabad. 2018;30(4):511-55.
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562-572. doi: 10.1056/NEJMra1608077
- Jasani B, Ismail A, Rao S, Patole S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants—A systematic review and meta‐analysis. Pediatr Pulmonol. 2018;53(7):987-992. doi: 10.1002/ppul.24014
- Kamath BD, MacGuire ER, McClure EM, Goldenberg RL, Jobe AH. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries. Pediatrics. 2011;127(6):1139-1146. doi: 10.1542/peds.2010-3212
- Clark RB, Davis SF, Visick MK, Bell RE. Feasibility of bubble continuous positive airway pressure in secondary facilities in low and middle income countries. Med Res Arch. 2018;6(5):1-8. doi: 10.18103/mra.v6i5.1779
- Myhre J, Immaculate M, Okeyo B, Anand M, Omoding A, Myhre L, et al. Effect of treatment of premature infants with respiratory distress using low-cost bubble CPAP in a rural African hospital. J Trop Pediatr. 2016;62(5):385-389. doi: 10.1093/tropej/fmw023
- Dewez JE, van den Broek N. Continuous positive airway pressure (CPAP) to treat respiratory distress in newborns in low-and middle-income countries. Trop Doct. 2017;47(1):19-22. doi: 10.1177/0049475516630210
- Ahmed FR, Jeergal NA, Channakeshava D, Reddy LN. Bubble continuous positive airway pressure as a primary modality of respiratory support in meconium aspiration syndrome. Indian J Child Health. 2019;6(12):669-672. doi: 10.32677/IJCH.2019.v06.i08.003
- Pillow JJ, Hillman N, Moss TJ, Polglase G, Bold G, Beaumont C, et al. Bubble continuous positive airway pressure enhances lung volume and gas exchange in preterm lambs. Am J Respir Crit Care Med. 2007;176(1):63-69. doi: 10.1164/rccm.200609-1368OC
- Chen A, Deshmukh AA, Richards-Kortum R, Molyneux E, Kawaza K, Cantor SB. Cost-effectiveness analysis of a low-cost bubble CPAP device in providing ventilatory support for neonates in Malawi–a preliminary report. BMC Pediatr. 2014;14(1):1-8. doi: 10.1186/s12887-014-0288-1
- Rezzonico R, Caccamo LM, Manfredini V, Cartabia M, Sanchez N, Paredes Z, et al. Impact of the systematic introduction of low-cost bubble nasal CPAP in a NICU of a developing country: a prospective pre-and post-intervention study. BMC Pediatr. 2015;15(1):1-9. doi: 10.1186/s12887-015-0338-3
- Thaddanee R, Chaudhari A, Chauhan H, Morbiwala S, Khilnani AK. Bubble continuous positive airway pressure machine versus Indigenous bubble continuous positive airway pressure as a respiratory support in preterm babies with respiratory distress syndrome: a prospective outcome research at a tertiary care centre in Gujarat, India. Int J Contemp Pediatr. 2018;5:493-498. doi: 10.18203/2349-3291.ijcp20180542
- Daga S, Mhatre S, Borhade A, Khan D. Home-made continuous positive airways pressure device may reduce mortality in neonates with respiratory distress in low-resource setting. J Trop Pediatr. 2014;60(5):343-347. doi: 10.1093/tropej/fmu023
- McAdams RM, Hedstrom AB, DiBlasi RM, Mant JE, Nyonyintono J, Otai CD, et al. Implementation of bubble CPAP in a rural Ugandan neonatal ICU. Respiratory care. 2015;60(3):437-445. doi: 10.4187/respcare.03438
- Ahmed Z, Shah SA, Khan UN, Subhani FA. Use of indigenously designed nasal bubble continuous positive airway pressure (NB-CPAP) in neonates with respiratory distress-experience from a military hospital. Pak Armed Forces Med. 2016;66(5):645-650.
- Rastogi S, Wong W, Gupta A, Bhutada A, Rastogi D. Gradual versus sudden weaning from nasal CPAP in preterm infants: a pilot randomized controlled trial. Respiratory care. 2013;58(3):511-516. doi: 10.4187/respcare.01999
- Kaur C, Sema A, Beri RS, Puliyel JM. A simple circuit to deliver bubbling CPAP. Indian Pediatr. 2008;45(4):312-314.
- Al-Lawama M, Alkhatib H, Wakileh Z, Elqaisi R, AlMassad G, Badran E, et al. Bubble CPAP therapy for neonatal respiratory distress in level III neonatal unit in Amman, Jordan: a prospective observational study. Int J Gen Med. 2019; 12: 25-30. doi: 10.2147/IJGM.S185264
- Arora V, Gediya SG, Jain R. Outcome of premature babies with RDS using bubble CPAP. Int J Contemp Pediatrics. 2017;4(3):939-942. doi: 10.18203/2349-3291.ijcp20171702
- Kakkilaya V, Wagner S, Mangona KL, Steven Brown L, Jubran I, He H, et al. Early predictors of continuous positive airway pressure failure in preterm neonates. J Perinatol. 2019;39(8):1081-1088. doi: 10.1038/s41372-019-0392-z
- Urs PS, Khan F, Maiya PP. Bubble CPAP-a primary respiratory support for respiratory distress syndrome in newborns. Indian Pediatr. 2009;46(5):409-411.
- Mustufa MA, Korejo R, Shahid A, Nasim S. Infection remains a leading cause of neonatal mortality among infants delivered at a tertiary hospital in Karachi, Pakistan. J Infect Dev Ctries. 2014;8(11):1470-1475. doi: 10.3855/jidc.3569
- Nahimana E, Ngendahayo M, Magge H, Odhiambo J, Amoroso CL, Muhirwa E, et al. Bubble CPAP to support preterm infants in rural Rwanda: a retrospective cohort study. BMC Pediatr. 2015;15(1):1-7. doi: 10.1186/s12887-015-0449-x
- Umran RM, Al-Musawi J. Effect of nasal bubble continuous positive airway pressure on neonatal mortality rate in Iraqi population. Kufa Med J. 2012;15(2):92-98.
- Soomro T, Tikmani SS. Success of Bubble CPAP in treatment of respiratory distress syndrome in preterm infants. J Gen Pract. 2016;4(4):1-4. doi: 10.4172/2329-9126.1000264
- Fatima T, Hamid MH, Jamshaid AA, Wasim A. Bubble nasal continuous positive airway pressure (bCPAP) versus control in neonates with respiratory distress. J Coll Physicians Surg Pak. 2020;10:16-17. doi: 10.29271/jcpsp.2020.08.805
- Bahman-Bijari B, Malekiyan A, Niknafs P, Baneshi MR. Bubble–CPAP vs. Ventilatory–CPAP in preterm infants with respiratory distress. Iran J Pediatr. 2011; 21(2): 151-158.
- Kawaza K, Machen HE, Brown J, Mwanza Z, Iniguez S. Efficacy of a low-cost bubble CPAP system in treatment of respiratory distress in a neonatal ward in Malawi. PLoS One;9(1):1-8. doi: 10.1371/journal.pone.0086327
This is an open-access article distributed under the terms of the CreativeCommons Attribution License (CC BY) 4.0 https://creativecommons.org/licenses/by/4.0/
